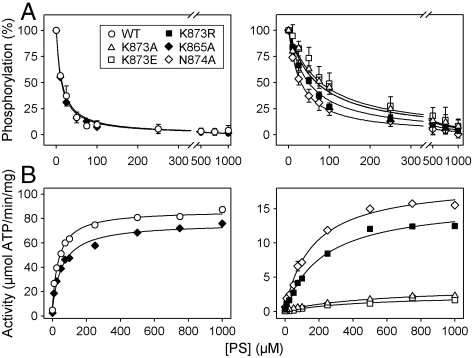
Fig. 3.
Apparent affinities of wild type and mutants for PS. (A) Following phosphorylation of PC reconstituted enzyme at 0 °C with [γ-32P]ATP in SPM containing CHAPS, PS dissolved in CHAPS was added at the indicated concentrations, and dephosphorylation was terminated 5 s later. K0.5 values for activation by PS (apparent PS affinities) were as follows: Wild type (12 μM), K865A (11 μM), K873A (88 μM), K873E (99 μM), K873R (46 μM), N874A (31 μM). (B) ATPase activity of wild type and mutants in the presence of CHAPS and PC with the indicated concentrations of PS. K0.5 values for activation by PS were as follows: Wild type (38 μM), K865A (58 μM), K873A (600 μM), K873E (490 μM), K873R (230 μM), N874A (170 μM). Refer to Table S2 for statistical analysis.