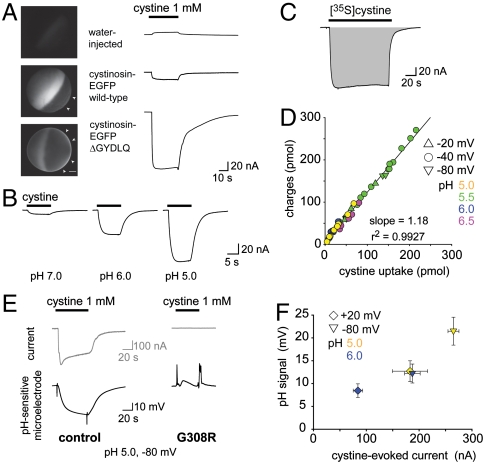
Fig. 2.
Cystinosin is a 1∶1 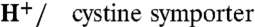 . (A) mRNA-injected oocytes were analyzed by epifluorescence microscopy and by two-electrode voltage clamp. Expression of EGFP-tagged, wild-type human cystinosin induces a low level of fluorescence at the oocyte plasma membrane (second micrograph from top, arrowheads) and a small inward current upon application of 1 mM extracellular cystine at pHout 5.0. Fluorescence at the plasma membrane and cystine-evoked current are dramatically increased after deletion of the lysosomal sorting motif GYDQL. The small outward current seen in water-injected oocytes is a mechanical artifact of the OpusXpress workstation, also observed when cystine-free buffer is applied. Scale bar, 0.2 mm. (B) An oocyte expressing human cystinosin-ΔGYDQL without EGFP tag was recorded at distinct pH values. (C and D) [35S]cystine was applied to 45 cystinosin-ΔGYDQL oocytes under diverse voltage and pHout conditions in two independent experiments. After recording, microelectrodes were removed and oocytes were counted by liquid scintillation. Time integral of cystine-evoked current (shaded area in C) is plotted in D as a function of [35S]cystine uptake. (E) Cystinosin translocates protons. Recording with a pH-sensitive microelectrode in the vicinity of a clamped cystinosin-ΔGYDQL-EGFP oocyte shows that the cystine-evoked inward current is associated with extracellular alkalinization. Both responses were abolished by the loss-of-function pathogenic mutation G308R. (F) Cystine-evoked alkalinization is proportional to the inward current. Means ± s.e.m. of 7 oocytes.
. (A) mRNA-injected oocytes were analyzed by epifluorescence microscopy and by two-electrode voltage clamp. Expression of EGFP-tagged, wild-type human cystinosin induces a low level of fluorescence at the oocyte plasma membrane (second micrograph from top, arrowheads) and a small inward current upon application of 1 mM extracellular cystine at pHout 5.0. Fluorescence at the plasma membrane and cystine-evoked current are dramatically increased after deletion of the lysosomal sorting motif GYDQL. The small outward current seen in water-injected oocytes is a mechanical artifact of the OpusXpress workstation, also observed when cystine-free buffer is applied. Scale bar, 0.2 mm. (B) An oocyte expressing human cystinosin-ΔGYDQL without EGFP tag was recorded at distinct pH values. (C and D) [35S]cystine was applied to 45 cystinosin-ΔGYDQL oocytes under diverse voltage and pHout conditions in two independent experiments. After recording, microelectrodes were removed and oocytes were counted by liquid scintillation. Time integral of cystine-evoked current (shaded area in C) is plotted in D as a function of [35S]cystine uptake. (E) Cystinosin translocates protons. Recording with a pH-sensitive microelectrode in the vicinity of a clamped cystinosin-ΔGYDQL-EGFP oocyte shows that the cystine-evoked inward current is associated with extracellular alkalinization. Both responses were abolished by the loss-of-function pathogenic mutation G308R. (F) Cystine-evoked alkalinization is proportional to the inward current. Means ± s.e.m. of 7 oocytes.