Figure 4.
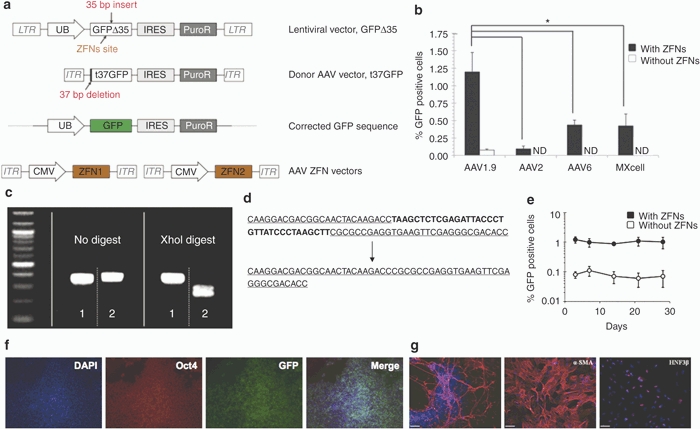
AAV1.9-mediated correction of a nonfunctional GFP expressed in hESCs. (a) Schematic overview depicting the targeting strategy for the GFP gene correction. The defective GFP gene—GFPΔ35, mutated by the insertion of a 35 bp fragment containing a translational stop codon13—was integrated into HSF-6 cells using a lentiviral vector. A targeting vector (t37GFP) containing a 5′ truncated GFP coding sequence was packaged into a recombinant AAV vector lacking a promoter. Homologous recombination between donor vector and integrated defective GFPΔ35 gene would correct the 35 bp mutation and result in fluorescent hESCs. (b) Gene targeting frequencies assessed as the percentage of GFP positive cells measured via flow cytometry 72 hours postinfection. Error bars indicate standard deviation (n = 3), *P < 0.01. ND, not detectable by flow cytometry. (c,d) Representative analyses of the targeted correction of GFPΔ35 gene. Genomic DNA from the “corrected” and “uncorrected” HSF-6 cells was amplified using PCR. The 35 bp mutation within the GFPΔ35 gene contains an Xho I site, and therefore only the PCR products from the “uncorrected” cells (sample 2) can be digested using Xho I, whereas the PCR products from the corrected cells (sample 1) show the restoration of the functional GFP gene without the Xho I site. DNA sequencing analysis also shows AAV1.9-mediated correction of the 35 bp mutation in HSF-6 cells originally expressing the mutated GFPΔ35 gene. (e) Time course of AAV-mediated GFPΔ35 correction in HSF-6 cells. Error bars indicate standard deviation (n = 3). (f) Maintenance of an undifferentiated state of gene-corrected, GFP-expressing cells for 30 days postinfection. GFP positive HSF-6 cells, isolated via FACS, were cultured for 30 days, then fixed and probed for the presence of GFP (green), Oct4 (red), and DAPI (blue). (g) Pluripotency of the HSF-6 cells carrying the corrected GFP gene was further confirmed through embryoid body-mediated in vitro differentiation to generate derivatives of all three germ layers, as indicated by the expression of the ectodermal marker β-III tubulin, the mesodermal marker α-smooth muscle actin (α-SMA), and the endodermal marker hepatocyte nuclear factor 3 β (HNF3β) after 24 days of differentiation (Bar = 100 µm). AAV, adeno-associated virus; CMV, cytomegalovirus; DAPI, 4′,6-diamidino-2-phenylindole; FACS, fluorescent-activated cell sorting; GFP, green fluorescent protein; hESC, human embryonic stem cell; IRES, internal ribosome entry site; ITR, inverted terminal repeat; LTR, long terminal repeat.
