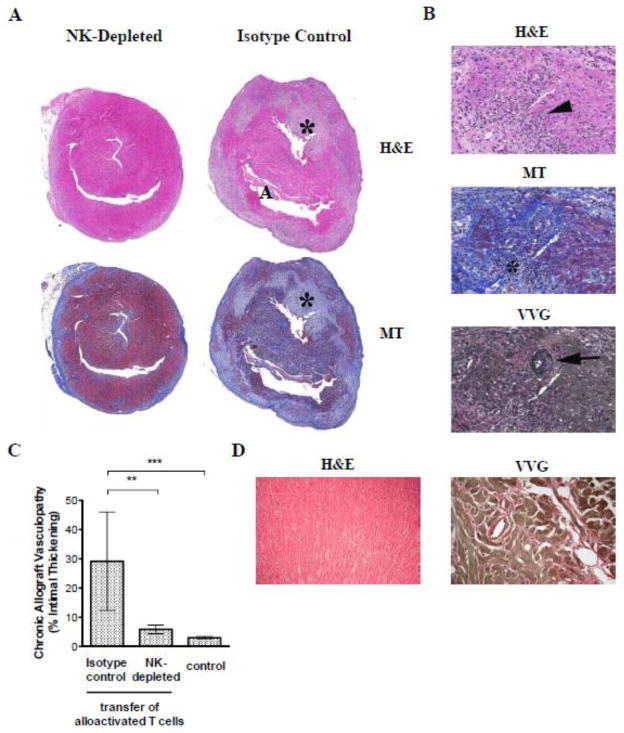
Figure 4. NK depletion reduces chronic rejection in a fully allogeneic transplantation model.
BALB/c cardiac allografts were parked for 50 days in splenectomized B6 aly/aly mice prior to the transfer of alloactivated T cells. Recipients were either NK-depleted during the parking period or remained NK-replete (isotype control). Grafts were harvested 70 – 100 days after T cell transfer and stained with H&E, MT, and VVG. (A) Representative low power micrographs of H&E and MT stained whole allograft sections of NK-depleted (left panel, n=6) and NK-replete mice (right panel, n=6) are shown. Note large areas of myocyte dropout (*) and early fibrosis (blue on MT stain) present in the NK-replete but not in the NK-depleted mouse. (B) Representative higher magnification (15x) of H&E, MT and VVG stained sections from NK-replete mice showing intense and widespread inflammation (arrowhead), evolving fibrosis (*) and obliterative with superimposed inflammatory arteritis (arrow). (C) Obliterative arteriopathy (ratio of intima to vessel wall) in intra-parenchymal vessels was quantitated by histomorphometry in NK-replete and NK-depleted mice (n = 6/group). Pooled results from control mice that either received no T cells (n=3) or mice that received naïve T cells 70 days following transplantation (n=4) are also shown. Data are mean ± SD. ** p<0.001, *** p<0.0001. (D) Representative low power micrographs of H&E and VVG stained allograft sections from control mice that received no T cells.