Abstract
Social interaction deficits and restricted repetitive behaviors and interests that characterize autism spectrum disorders (ASDs) may both reflect aberrant functioning of brain reward circuits. However, no neuroimaging study to date has investigated the integrity of reward circuits using an incentive delay paradigm in individuals with ASDs. In the present study, we used functional magnetic resonance imaging to assess blood-oxygen level-dependent activation during reward anticipation and outcomes in 15 participants with an ASD and 16 matched control participants. Brain activation was assessed during anticipation of and in response to monetary incentives and object image incentives previously shown to be visually salient for individuals with ASDs (e.g. trains, electronics). Participants with ASDs showed decreased nucleus accumbens activation during monetary anticipation and outcomes, but not during object anticipation or outcomes. Group × reward-type-interaction tests revealed robust interaction effects in bilateral nucleus accumbens during reward anticipation and in ventromedial prefrontal cortex during reward outcomes, indicating differential responses contingent on reward type in these regions. Results suggest that ASDs are characterized by reward-circuitry hypoactivation in response to monetary incentives but not in response to autism-relevant object images. The clinical implications of the double dissociation of reward type and temporal phase in reward circuitry function in ASD are discussed.
Keywords: autism, reward, nucleus accumbens, anticipation, functional magnetic resonance imaging
INTRODUCTION
Social interaction deficits and restricted repetitive behaviors and interests are core features of autism spectrum disorders (ASDs; American Psychiatric Association, 1994). A novel approach to understanding the pathophysiology of these core autism symptoms is to assess the functional integrity of brain reward circuits. It may be the case that hypo-responsivity to social stimuli in ASD reflects a failure to assign reward value to social interactions (Mundy and Neal, 2001; Dawson et al., 2004, 2005), a conceptualization supported by data demonstrating a disturbance in the motivational mechanisms that normally draw an infant’s attention to social information (Rochat and Striano, 1999). Similarly, it may be the case that restricted repetitive behaviors and interests reflect hyper-reactive reward brain-circuitry responses to certain classes of stimuli in individuals with ASD, a model that may help to explain symptoms of circumscribed interests that are highly prevalent in ASDs (Klin et al., 2007; Lam et al., 2008).
In non-clinical contexts, anticipation of rewarding stimuli recruits the nucleus accumbens (NAc) as well as other limbic structures including the caudate, thalamus and putamen, markers of incentive motivation underlying approach behaviors to salient goals. The experience of pleasure, on the other hand, activates the NAc as well as the caudate, putamen, amygdala, and, perhaps most robustly, the ventromedial prefrontal cortex (VMPFC; Knutson et al., 2001; Ernst et al., 2004; Wacker et al., 2009). Although most studies of reward processing have used monetary incentives, reactivity of these brain circuits has been demonstrated in response to a range of stimuli, including pleasant pictures (Canli et al., 2001), appetizing foods (Stice et al.), juice (Kim et al., 2010) and pictures of attractive faces (Hayden et al., 2007; Winston et al., 2007).
Neurobiological responses to rewards have been shown to be aberrant in a number of psychiatric disorders, including major depressive disorder (Knutson et al., 2008), schizophrenia (Juckel et al., 2006), substance dependence (Wrase et al., 2007) and attention-deficit/hyperactivity disorder (Strohle et al., 2008). However, despite the potential centrality of reward-circuitry function to core autism symptoms, no brain imaging study to date has examined responses to the anticipation and receipt of rewarding stimuli in ASDs. Although one ASD study examined responses to a task that involved monetary rewards (Schmitz et al., 2008), the effects of reward feedback were assessed during a sustained attention task, rather than responses to rewards per se. Additionally, Scott-Van Zeelan et al. (2010) investigated rewarded implicit learning in children with ASDs using social and monetary rewards, and found diminished ventral striatal response during social, but not monetary, rewarded learning. However, this study did not assess responses during reward anticipation.
In the present study, we used a monetary incentive delay (MID; Knutson et al., 2001) task to assess brain responses during reward anticipation and outcomes in individuals with ASDs via functional magnetic resonance imaging (fMRI). Additionally, to test whether reward circuitry function in ASDs is moderated by different classes of reward stimuli, brain function was assessed in response to both monetary incentives and object images known to be visually salient for individuals with ASDs (Sasson et al., 2008, 2010), conceptualized as a proxy for circumscribed interests that are highly prevalent in ASDs (South et al., 2005; Klin et al., 2007; Lam et al., 2008).
Primary hypotheses concerned responses to monetary incentives and were based on theories postulating that social amotivation in ASDs may reflect broader hypo-responsivity of neurobiological systems that respond to rewards (Dawson et al., 2004, 2005). We hypothesized that participants with ASDs would demonstrate decreased activation in the striatum, and in the NAc in particular, during monetary anticipation, and decreased striatal and VMPFC activation during monetary outcomes. Secondary hypotheses concerned responses to object images and were based on previous studies of circumscribed interests in ASDs that have shown bias towards these categories of stimuli (Turner, 1999; South et al., 2005; Klin et al., 2007; Sasson et al., 2008; Sasson et al., 2010; Turner-Brown et al., 2010). We hypothesized that group differences in NAc and VMPFC activation during the anticipation and outcome phases of the task, respectively, would be moderated by reward type (i.e. monetary incentives vs object images), reflecting relatively greater reward circuitry activation within the ASD group in response to object images relative to monetary incentives. Exploratory analyses evaluated potential linkages between the severity of autism symptoms and brain activation magnitudes to both types of incentives.
MATERIALS AND METHOD
Participants
Sixteen right-handed adult male control participants (27.5 ± 7.5-years old) were recruited from lists of control samples maintained by the Duke-UNC Brain Imaging and Analysis Center. Control participants were not taking any psychotropic medications at the time of scanning. Fifteen adult males with ASDs identified as high-functioning comprised the ASD group (30.1 ± 11.6-years old; 14 right-handed; two diagnosed with Asperger’s Disorder and 13 with High Functioning Autism) and were recruited via the Autism Subject Registry maintained through the UNC Carolina Institute for Developmental Disabilities. Exclusion criteria for the ASD group included a history of medical conditions associated with autism, including Fragile X syndrome, tuberous sclerosis, neurofibromatosis, phenylketouria, epilepsy and gross brain injury, full-scale intelligence <80 or MRI contraindications. Six ASD participants were not taking any psychotropics; three were taking citalopram, one was taking prozac, one was taking risperdol and the remaining four were taking multiple psychotropic medications (i.e. combinations of prozac, lorazepam, clonidine, zyprexa, zoloft and abilify).
Diagnoses of ASDs were based on a history of clinical diagnosis confirmed by proband assessment by a research reliable assessor via the Autism Diagnostic Observation Schedule-Generic (ADOS-G; Lord et al., 2000) with standard clinical algorithm cutoffs. Participants consented to a protocol approved by the local Human Investigations Committees at both UNC-Chapel Hill and Duke University Medical Center and were paid at least $35 for scanning. Participants had normal or corrected-to-normal vision and completed a mock scan session prior to imaging.
Clinical testing
Participants completed: (i) the Weschler Abbreviated Scale of Intelligence (WASI) (Weschler, 1999) [one ASD participant completed the Leiter-R (Roid and Miller, 1997) instead of the WASI]; (ii) the Repetitive Behavior Scale-Revised (RBS-R) (Bodfish et al., 1999; Lam and Aman, 2007), a measure designed to assess multiple RRB factors; and (iii) the Autism Quotient (Baron-Cohen et al., 2001), administered to assess the overall severity of autism symptoms as well as to verify that the control group did not have significant autistic symptoms. When possible, intelligence testing records of ASD participants were accessed, with permission of participants, from records of past studies conducted at UNC-Chapel Hill (n = 6). All other assessments were conducted on the day of the fMRI scan.
fMRI task
The fMRI task was modified from the MID task as implemented in Knutson et al. (2000). Participants completed six functional imaging runs. Three runs were the standard ‘win version’ of the MID task during which money could be won or not won, but could not be lost. Three runs were modified so that participants could ‘win’ the opportunity to view a salient object image rather than money (the procedure for deriving these images is described below). Run types (i.e. ‘money runs’ or ‘object runs’) were presented in alternating and counter-balanced order. Runs began with a 10-s instructional screen indicating the forthcoming run type. The two reward types were segregated by run to minimize the number of cues to be memorized.
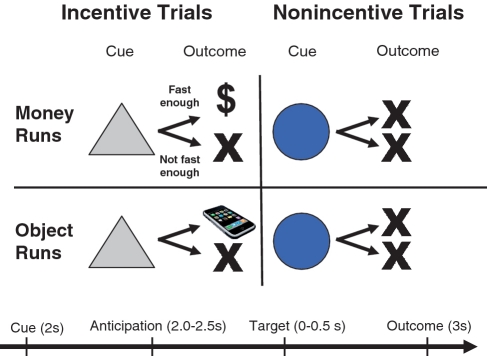
Task conditions and trial timings are summarized in Figure 1. Each trial consisted of: (i) a 2000-ms cue (either a triangle or a circle) that indicated whether adequately quick responses to a forthcoming bulls-eye would result in a win or a non-win; (ii) a 2000–2500 ms crosshair fixation; (iii) a target bulls-eye, presented for up to 500 ms, that required a speeded button press; (iv) 3000 ms of feedback that indicated whether that trial was a win or a non-win; and (v) a variable length ITI crosshair presented such that the total duration of each trial was 12 s. Trial types (i.e. potential win or non-win) were aperiodic and pseudorandomly ordered. Each 8-min run contained 40 trials: 20 were potential win trials, 20 were potential non-win trials.
Fig. 1.
Modified MID task. ‘Money’ and ‘Object’ runs were presented in alternating order. Each trial consisted of a cue (i.e. a triangle indicated an incentive trial, a circle indicated a non-incentive trial), an anticipatory delay, a target and feedback.
During money runs, participants won $1 per trial for an adequately quick response to the forthcoming bulls-eye. During object image runs, participants ‘won’ one image per trial for an adequately quick response to the forthcoming bulls-eye. Feedback was given to indicate whether each trial was a ‘win’ or not as well as cumulative win totals. Participants were instructed to respond to all target bulls-eyes as quickly as possible, that win or non-win outcomes were contingent on reaction times (RTs), and that the amount of money they would receive for participating in the imaging portion of the study was contingent on their monetary win totals during the scanner task. They were also told that adequately quick responses on object trials would enable them to view an object on those trials and that they would receive a high-quality printout of the object images ‘won’. The task was adaptive such that participants were successful on two-thirds of trials, regardless of individual differences in RTs. Participants viewed a booklet of all object images prior to the scanning session. Stimuli were presented using E-Prime presentation software v. 1.1 (Psychology Software Tools Inc., Pittsburgh, PA, USA) and displayed through magnet-compatible goggles (Resonance Technology Inc., Northridge CA, USA).
Object incentives
An ASD-relevant set of object images has been systematically derived by our research group in the following manner. First, a large number of potential object images was selected based on profiles of responses from semi-structured parent-report interviews about circumscribed interests in ASDs (e.g. trains and electronic devices; South et al., 2005; Klin et al., 2007). The validity of the content of these object images was confirmed by recent findings that, among 50 children with ASDs and 50 children without ASDs, parents reported that the content of interests within the ASD group was significantly more likely to involve machines, mechanical systems, vehicles, building, computers, physics and object motions (Turner-Brown et al., 2011). Next, the set of object images was evaluated via a passive-viewing visual exploration eyetracking study of 29 children with and 24 children without ASDs (Sasson et al., 2008; see also Sasson et al., 2010). This eye-tracking study-identified object images that garnered relatively greater visual attention (i.e. numbers of fixations) in the ASD sample. The 40 object images that demonstrated the highest number of visual fixations were used in this study (Appendix Fig. A1). Object images were subsequently modified to a resolution of 300 pixels per inch, a width of 500 pixels, a height of 400 pixels and superimposed on a black background.
Imaging methods
Scanning was performed on a General Electric Health Technologies, 3 Tesla Signa Excite HD scanner system with 50-mT/m gradients (General Electric, Waukesha, WI, USA). Head movement was restricted using foam cushions. An eight-channel head coil was used for parallel imaging. Thirty high-resolution images were acquired using a 3D fast SPGR pulse sequence (TR = 7.332 ms; TE = 3.032 ms; FOV = 22 cm; image matrix = 2562; voxel size = 0.859384 × 0.859375 × 3.800000 mm) and used for coregistration with the functional data. These structural images were aligned in the near axial plane defined by the anterior and posterior commissures. Whole brain functional images consisted of 30 slices parallel to the AC-PC plane using a BOLD-sensitive gradient-echo EPI sequence, at TR of 2000 ms (TE: 30 ms; FOV: 22 cm; isotropic voxel size: 3.4375 × 3.4375 × 4.0000). Runs began with four discarded RF excitations to allow for steady state equilibrium.
Imaging data analysis
Functional data were preprocessed using FSL version 4.0.4 [Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, UK]. Timing files were converted to FSL compatible format and NIFTI image data files were generated. Preprocessing was applied in the following steps: (i) brain extraction for non-brain removal (Smith et al., 2004); (ii) motion correction using MCFLIRT (Smith, 2002); (iii) spatial smoothing using a Gaussian kernel of FWHM 5 mm; (iv) mean-based intensity normalization of all volumes by the same factor; and (v) high-pass filtering (Jenkinson et al., 2002). Functional images of each participant were co-registered to structural images in native space, and structural images were normalized into a standard stereotaxic space (Montreal Neurological Institute) for intersubject comparison. The same transformation matrices used for structural-to-standard transformations were then used for functional-to-standard space transformations of co-registered functional images. All registrations were carried out using an intermodal registration tool (Jenkinson et al., 2002; Smith et al., 2004). Voxel-wise temporal autocorrelation was estimated and corrected using FMRIB’s Improved Linear Model (Jenkinson and Smith, 2001).
Event onset times were used to model a signal response containing a regressor for each response type, which was convolved with a double-γ function to model the hemodynamic response. Model fitting generated whole brain images of parameter estimates and variances, representing average signal change from baseline (activation; positive regressor) and below baseline (deactivation; negative regressor). Group-wise activation images were calculated by a mixed effects higher level analysis using Bayesian estimation techniques, FMRIB Local Analysis of Mixed Effects (FILM; Woolrich et al., 2001). Following the guidelines of Lieberman and Cunningham (2009) for clinical studies where a balance of Types I and II error probabilities are sought, clusters of ten or more voxels with minimum Z-values of >2.50 (P < 0.005) were identified using customized MATLAB scripts.
Imaging data analytic strategy
Anticipation and outcome phases were analyzed separately. For both phases, the primary method of analysis was to evaluate clusters that revealed a significant effect of diagnostic status on the contrast of potential win versus non-win trials during the anticipation phase and for wins vs non-wins during the outcome phase. This approach was carried out separately for monetary and object incentive runs. Next, a higher order 2 (Group: ASD, control) × 2 (Reward Type: money, object images) interaction model was tested to evaluate group differences with respect to reward type during both phases. Activation localizations were based on Harvard–Oxford cortical and subcortical structural probabilistic atlases as implemented in FSLView v3.0.
RESULTS
Sample characteristics
Table 1 illustrates that diagnostic groups did not differ on the full scale or verbal measures of IQ but differed with respect to performance IQ. Results of analyses that included performance IQ as a covariate were nearly identical. Groups differed in the ‘sameness behavior’ and ‘circumscribed interests’ subscales of the RBS-R, as well as in overall autism symptoms as measured by the AQ.
Table 1.
Means (SDs) of demographic data and symptom profiles
| Autism (n = 15) | Control (n = 16) | t(P) | |
|---|---|---|---|
| Age | 30.1 (11.6) | 27.5 (7.5) | 0.75 (0.46) |
| WASIa | |||
| Verbal | 109.9 (26.2) | 117.9 (13.4) | 1.08 (0.29) |
| Performance | 111.0 (15.9) | 121.8 (7.7) | 2.41 (0.023) |
| Full | 111.9 (22.7) | 122.2 (10.7) | 1.57 (0.13) |
| AQb | 26.1 (10.2) | 14.8 (6.4) | 3.90 (0.00064) |
| ADOS | |||
| Comm | 5.1 (4.5) | ||
| SI | 8.3 (2.6) | ||
| SBRI | 2.2 (1.6) | ||
| RBS-R | |||
| Stereo | 2.9 (2.9) | 2.4 (2.5) | 0.55 (0.59) |
| SIB | 1.9 (0.9) | 0.9 (1.2) | 1.24 (0.23) |
| Comp | 5.5 (2.9) | 2.9 (4.3) | 1.52 (0.14) |
| RIT | 5.2 (2.2) | 2.2 (3.5) | 1.95 (0.061) |
| Same | 7.9 (3.8) | 3.8 (3.8) | 2.20 (0.036) |
| CI | 2.8 (1.1) | 1.1 (1.1) | 2.45 (0.021) |
| Total | 25.8 (19.6) | 13.8 (13.3) | 1.94 (0.062) |
aWASI missing from one autism participant with Leiter IQ score of 121.
bAQ missing from two autism and two control participants.
WASI, Wechsler Abbreviated Scale of Intelligence (Weschler, 1999); ADOS, Autism Diagnostic Observation Scale (Lord et al., 2000); Comm, Communication; SI, Reciprocal Social Interaction; SBRI, stereotyped behaviors and restricted interests; AQ, Autism Spectrum Quotient (Baron-Cohen et al., 2001; a threshold of 32 or higher suggests cause for clinical concern in community samples); RBS-R, Repetitive Behavior Scale-Revised (Bodfish et al., 1999; Lam and Aman, 2007); Stereo, stereotyped behavior; SIB, self-injurious behavior; Comp, compulsive behavior; Rit, ritualistic behavior; Same, sameness behavior; CI, circumscribed interests.
MID RTs
Average RTs to bulls-eyes presented within the MID task were compared via a 2 (Group: ASD, neurotypical) × 2 (Reward Type: Money, object image) rMANOVA that revealed a main effect of Reward Type, multivariate F(1, 29) = 9.14, P < 0.006, reflecting that RTs overall were faster to money (average = 201 ± 37 ms) than image (average = 212 ± 39 ms) bulls-eyes, and a main effect of Group, F(1, 29) = 4.34, P < 0.05, reflecting that the neurotypical group (average = 193 ± 29 ms) was faster overall than the ASD group (average = 219 ± 39 ms), but no Group X Reward Type interaction, multivariate F(1, 29) = 1.30, P > 0.26.
Monetary incentive fMRI results
Anticipation phase
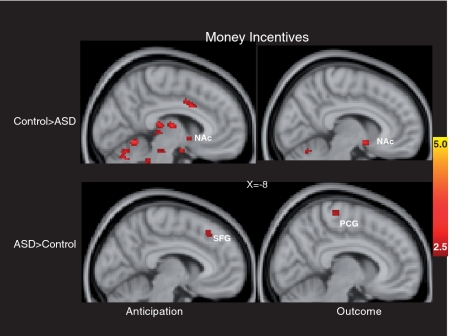
The left side of Figure 2 and the top of Table 2 depict whole-brain results during monetary anticipation. The figure illustrates that striatal reward regions, including the left NAc and right putamen, were less active in the ASD relative to the control group. In contrast, there were no striatal regions that showed relatively greater activation in the ASD group relative to the control group.
Fig. 2.
Brain areas showing significant group differences in response to monetary incentives. Anticipatory responses are on the left and outcome responses are on the right; clusters with relatively greater activation in the control group are in the top panels, clusters with relatively greater activation in the ASD group are in the bottom panels. SFG: superior frontal gyrus; PCG: precentral gyrus.
Table 2.
Clusters showing significant group differences in response to monetary incentives
| Region | Side | Size (mm3) | Z (maximum) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Anticipation | ||||||
| Control > autism | ||||||
| Accumbens | Left | 104 | 2.72 | −6 | 6 | −4 |
| Anterior cingulate gyrus | Left | 1424 | 3.15 | −4 | 18 | 26 |
| Cerebelluma | Left | 360 | 2.87 | −36 | −60 | −26 |
| Cerebelluma | Right | 472 | 3.05 | 8 | −44 | −28 |
| Frontal operculum cortex | Right | 152 | 2.79 | 40 | 20 | 4 |
| Frontal orbital cortex | Left | 104 | 2.68 | −22 | 8 | −20 |
| Insular cortex | Left | 1848 | 3.76 | −38 | 14 | −12 |
| Insular cortex | Right | 280 | 2.95 | 40 | 6 | −2 |
| Lingual gyrus | Left | 4640 | 3.32 | −8 | −58 | −8 |
| Occipital pole | Right | 600 | 3.14 | 14 | −92 | −6 |
| Planum polare | Right | 136 | 2.82 | 44 | −14 | −6 |
| Precentral gyrus | Left | 104 | 2.85 | −30 | −16 | 70 |
| Putamen | Right | 464 | 2.99 | 30 | −14 | −10 |
| Temporal polea | Right | 272 | 3.07 | 38 | 18 | −24 |
| Autism > control | ||||||
| Angular gyrus | Left | 3768 | 3.61 | −50 | −52 | 24 |
| Frontal pole | Left | 80 | 2.83 | −20 | 56 | 30 |
| Hippocampus | Right | 144 | 2.89 | 26 | −14 | −26 |
| Middle-frontal gyrus | Left | 480 | 3.04 | −36 | 22 | 44 |
| Middle-temporal gyrus (temporooccipital) | Left | 88 | 2.60 | −64 | −50 | 0 |
| Postcentral gyrus | Right | 528 | 4.06 | 50 | −20 | 60 |
| Precuneous cortex | Left | 112 | 2.75 | −4 | −60 | 46 |
| Superior frontal gyrus | Left | 1016 | 3.52 | −12 | 26 | 52 |
| Superior temporal gyrus (posterior) | Left | 88 | 2.79 | −58 | −30 | −2 |
| Superior temporal gyrus (posterior) | Right | 136 | 3.15 | 66 | −16 | −4 |
| Outcome | ||||||
| Control > autism | ||||||
| Accumbens | Left | 88 | 2.96 | −8 | 8 | −10 |
| Frontal pole | Right | 280 | 3.58 | 48 | 34 | −12 |
| Insular cortex | Right | 88 | 2.74 | 44 | 6 | −10 |
| Autism > control | ||||||
| Frontal pole | Left | 752 | 3.45 | −44 | 46 | 12 |
| Hippocampus | Right | 520 | 3.18 | 26 | −10 | −26 |
| Inferior frontal gyrus (pars opercularis)a | Left | 536 | 3 | −44 | 28 | 6 |
| Juxtapositional lobule cortex | Left | 80 | 2.63 | 0 | 6 | 62 |
| Lateral occipital cortex (inferior) | Right | 112 | 2.73 | 40 | −64 | 0 |
| Lateral occipital cortex (superior)a | Left | 1312 | 4 | −32 | −76 | 48 |
| Lateral occipital cortex (superior) | Right | 216 | 2.99 | 30 | −66 | 28 |
| Middle-frontal gyrus | Left | 2272 | 4.06 | −36 | 38 | 38 |
| Middle-temporal gyrus (posterior) | Left | 176 | 2.81 | −64 | −24 | −10 |
| Middle-temporal gyrus (temporooccipital) | Left | 128 | 2.8 | −64 | −50 | −4 |
| Postcentral gyrus | Left | 592 | 2.84 | −44 | −26 | 54 |
| Postcentral gyrus | Right | 520 | 3.38 | 42 | −24 | 52 |
| Precentral gyrusb | Left | 2488 | 3.22 | −34 | −4 | 56 |
| Precentral gyrusc | Right | 136 | 3.51 | 30 | −16 | 58 |
| Superior frontal gyrusa | Left | 2024 | 4.59 | −14 | 28 | 58 |
| Superior frontal gyrus | Right | 264 | 2.96 | 4 | 20 | 58 |
| Superior parietal lobule | Left | 256 | 3.12 | −36 | −52 | 64 |
| Superior temporal gyrus (posterior) | Left | 88 | 2.78 | −68 | −36 | 8 |
aTwo clusters within same region, coordinates and peak activation reported for highest peak activation.
bFour clusters within same region, coordinates and peak activation reported for highest peak activation.
cThree clusters within same region, coordinates and peak activation reported for highest peak activation.
Outcome phase
The right side of Figure 2 and the bottom of Table 2 depict whole-brain results during monetary outcomes. The figure illustrates that the ASD group demonstrated relatively less activation in the left NAc, as well as the right frontal pole and right insular cortex, but not in VMPFC. In contrast, the ASD group did not demonstrate relatively increased activation in the VMPFC or in any striatal regions, although the ASD group demonstrated relatively greater activation in a number of cortical regions outside of classic reward circuits.
Object incentive fMRI results
Anticipation phase
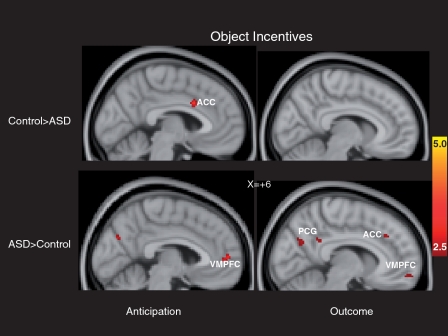
The left side of Figure 3 and the top of Table 3 depict whole-brain results during anticipation of object incentives. In this context, the ASD group did not show decreased activation in striatal regions, but rather demonstrated decreased activation in the dorsal anterior cingulate cortex only, relative to the control group. The ASD group demonstrated relatively increased activation in clusters of bilateral paracingulate gyrus as well other frontal lobe regions.
Fig. 3.
Brain areas showing significant group differences in response to object incentives. Anticipatory responses are on the left and outcome responses are on the right; clusters with relatively greater activation in the control group are in the top panels, clusters with relatively greater activation in the ASD group are in the bottom panels. ACC: anterior cingulate cortex; PCG: posterior cingulate gyrus.
Table 3.
Clusters showing significant group differences in response to object incentives
| Region | Side | Size (mm3) | Z (maximum) | MNI coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Anticipation | ||||||
| Control > autism | ||||||
| Anterior cingulate gyrus | Left | 152 | 2.86 | −4 | 12 | 32 |
| Autism > control | ||||||
| Angular gyrus | Right | 480 | 3.28 | 54 | −56 | 20 |
| Frontal pole | Left | 456 | 3.08 | −46 | 36 | −2 |
| Frontal pole | Right | 88 | 2.72 | 26 | 40 | 44 |
| Lateral occipital cortex (inferior) | Left | 552 | 3.63 | −40 | −64 | 14 |
| Middle-frontal gyrus | Left | 552 | 3.35 | −26 | 36 | 36 |
| Middle-temporal gyrus (posterior) | Right | 88 | 2.83 | 62 | −22 | −6 |
| Paracingulate gyrus | Left | 160 | 2.84 | −4 | 54 | 8 |
| Paracingulate gyrus (VMPFC) | Right | 216 | 3.07 | 6 | 54 | 6 |
| Postcentral gyrus | Left | 80 | 2.85 | −34 | −34 | 42 |
| Postcentral gyrusa | Right | 808 | 3.55 | 44 | −30 | 56 |
| Precentral gyrusb | Right | 560 | 3.15 | 48 | −10 | 56 |
| Precuneous cortexc | Left | 200 | 2.81 | −6 | −72 | 30 |
| Superior temporal gyrus (posterior) | Right | 128 | 2.95 | 60 | −22 | 2 |
| Outcome | ||||||
| Control > autism | ||||||
| Inferior frontal gyrus (pars opercularis) | Left | 144 | 3.1 | −40 | 16 | 22 |
| Lateral occipital cortez (superior) | Left | 152 | 3.09 | −36 | −84 | 24 |
| Occipital fusiform gyrusc | Left | 608 | 3.01 | −18 | −80 | −6 |
| Occipital pole | Left | 88 | 2.78 | −24 | −94 | 2 |
| Superior parietal lobule | Right | 96 | 2.81 | 18 | −52 | 72 |
| Temporal occipital fusiform cortex | Left | 128 | 2.81 | −26 | −52 | −12 |
| Autism > control | ||||||
| Angular gyrus | Left | 640 | 3.18 | −58 | −56 | 12 |
| Cingulate gyrus (posterior) | Right | 152 | 2.98 | 10 | −44 | 28 |
| Frontal orbital cortex | Left | 408 | 3.65 | −26 | 16 | −26 |
| Frontal pole (VMPFC) | Right | 152 | 2.73 | 6 | 56 | −10 |
| Frontal pole | Right | 320 | 3.19 | 28 | 38 | 42 |
| Frontal pole | Left | 104 | 2.76 | −24 | 62 | −8 |
| Lateral occipital cortex (superior) | Left | 336 | 2.96 | −38 | −64 | 16 |
| Middle-temporal gyrus (posterior) | Left | 80 | 2.96 | −58 | −14 | −14 |
| Middle-temporal gyrus (temporooccipital) | Right | 528 | 3.08 | 66 | −46 | 2 |
| Planum temporale | Left | 80 | 2.89 | −62 | −24 | 10 |
| Precuneus cortex | Right | 1040 | 3.21 | 10 | −66 | 28 |
| Paracingulate gyrus | Right | 128 | 3.03 | 6 | 30 | 36 |
aThree clusters within same region, coordinates and peak activation reported for highest peak activation.
bFour clusters within same region, coordinates and peak activation reported for highest peak activation.
cTwo clusters within same region, coordinates and peak activation reported for highest peak activation.
Outcome phase
The right side of Figure 3 and the bottom of Table 3 depict whole-brain results while viewing salient object incentives. The ASD group did not show decreased activation in any striatal regions or ventromedial prefrontal regions, relative to the control group. The ASD did demonstrate, however, relatively increased activation in VMPFC (Brodmann’s area 10) as well as other frontal lobe regions relative to the control group.
Group × reward type fMRI results
Anticipation phase
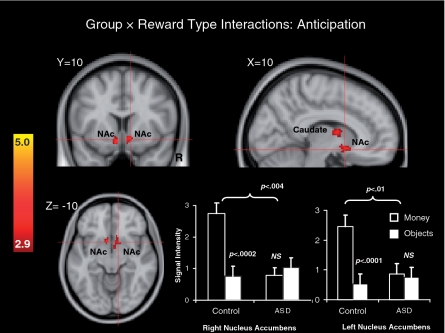
Figure 4 and the top of Table 4 depict whole-brain 2 (Group: ASD, control) × 2 (Reward Type: money, objects) interaction terms for anticipatory phase data. This analysis revealed clusters in bilateral NAc, as well as the left caudate nucleus, cerebellum, left-frontal orbital cortex, left lingual gyrus, right occipital pole, left planum temporale, left temporal pole and left thalamus. To further examine the patterns of data within the left and right NAc clusters identified by significant interaction terms, average signal intensities were extracted from these two clusters, grouped by diagnosis and reward type (see the lower right of Figure 4). Mixed 2 (Group: ASD, control) × 2 (Reward Type: money, objects) MANOVAs performed separately on signal intensity Z-values in the right and left NAc revealed significant Group × Reward Type interactions in both left and right NAc, multivariate F’s (1, 29) > 18.73, P’s < 0.0002, significant main effects of Reward Type, multivariate F’s (1, 29) > 11.14, P’s < 0.003 and trends towards a main effects of Group, F’s (1, 29) < 13.56, P’s < 0.07. Follow-up within-groups t-tests revealed a significantly greater response to money than objects in the control group, P < 0.0002 (right NAc) and P < 0.0001 (left NAc), but no reward type difference in the ASD group, P’s > 0.40. Between-groups t-tests revealed significantly greater responses to money in the control relative to the ASD group, P < 0.004 (right NAc) and P < 0.01 (left NAc), but no significant difference between groups in responses to objects, P’s > 0.45. These results suggest that in both the left and right NAc, difference between groups with respect to anticipatory responses to rewards were contingent on reward type, and that responses to monetary rewards in the control group were larger than in the other conditions.
Fig. 4.
Brain areas showing significant Group × Reward Type interactions during anticipation and bar graphs depicting Z-score intensity values in the right and left NAc clusters identified by significant interaction effects. Error bars represent standard errors of the mean.
Table 4.
Clusters showing significant Group (Control, ASD) × Reward Type (Money, Object) interactions
| Region | Side | Size (mm3) | Z (maximum) | MNI Coordinates |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Anticipation | ||||||
| Accumbens | Left | 624 | 3.61 | −6 | 18 | −14 |
| Accumbens | Left | 992 | 3.71 | 0 | 2 | −6 |
| Caudate nucleus | Left | 40 | 3.06 | −18 | 22 | 10 |
| Caudate nucleus | Left | 160 | 3.20 | −16 | 12 | 16 |
| Cerebellum | Right | 64 | 3.12 | 28 | −52 | −30 |
| Cerebelluma | Right | 744 | 3.5 | 4 | −66 | −14 |
| Frontal orbital cortex | Left | 72 | 3.3 | −32 | 24 | −22 |
| Lingual gyrus | Left | 96 | 3.5 | −2 | −84 | −18 |
| Occipital pole | Right | 40 | 3.05 | 12 | −92 | −6 |
| Planum temporale | Left | 128 | 3.33 | −42 | −40 | 12 |
| Temporal pole | Left | 120 | 3.57 | −534 | 12 | −12 |
| Thalamus | Left | 5656 | 3.89 | −14 | −8 | 10 |
| Outcome | ||||||
| Accumbens | Right | 448 | 2.71 | 8 | 8 | −8 |
| Angular gyrus | Right | 400 | 2.42 | 56 | −42 | 38 |
| Cerebellumb | Right | 1320 | 2.53 | 4 | −60 | −20 |
| Cerebelluma | Left | 360 | 2.73 | −14 | −66 | −28 |
| Cuneal cortex | Right | 400 | 2.57 | 12 | −68 | 22 |
| Frontal orbital cortex | Right | 104 | 2.53 | 36 | 28 | 4 |
| Frontal orbital cortex | Right | 704 | 3.04 | 50 | 34 | −12 |
| Frontal pole | Left | 40 | 2.29 | −28 | −60 | 12 |
| Frontal pole | Right | 256 | 2.68 | 8 | 56 | −14 |
| Frontal pole | Right | 320 | 2.70 | 26 | 38 | 42 |
| Heschl’s gyrus | Left | 288 | 2.45 | −40 | −22 | 6 |
| Insular cortex | Left | 144 | 2.26 | −36 | 16 | −10 |
| Insular cortex | Right | 48 | 2.11 | 42 | 16 | −8 |
| Lingual gyrus | Right | 360 | 2.88 | 2 | −82 | −24 |
| Middle-frontal gyrus | Right | 136 | 2.48 | 34 | 18 | 54 |
| Middle-temporal gyrus, temporooccipitala | Right | 328 | 2.55 | 62 | −52 | 4 |
| Paracingulate gyrus | Right | 120 | 2.42 | 8 | 30 | 40 |
| Paracingulate gyrus (VMPFC) | Right | 1544 | 2.70 | 0 | 42 | −6 |
| Planum polare | Left | 360 | 2.62 | −44 | 8 | −20 |
| Precuneous cortex | Left | 72 | 2.49 | −16 | −68 | 24 |
| Precuneous cortex | Right | 40 | 2.31 | 6 | −80 | 46 |
| Supramarginal gyrus (posterior) | Right | 88 | 2.24 | 60 | −36 | 18 |
| Temporal pole | Right | 48 | 2.18 | 42 | 18 | −20 |
| Thalamus | Right | 184 | 2.35 | 2 | −16 | 10 |
aTwo clusters within same region, coordinates and peak activation reported for highest peak activation.
Outcome phase
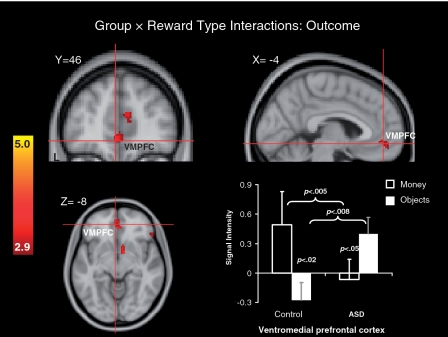
Figure 5 and the bottom of Table 4 depict whole-brain 2 (Group: ASD, control) × 2 (Reward Type: money, objects) interaction terms for outcome data. This analysis revealed a significant cluster in VMPFC, as well as the right NAc and a number of other prefrontal regions. A mixed 2 (Group: ASD, control) × 2 (Reward Type: money, objects) MANOVA performed on signal intensity Z-values within the VMPFC cluster identified by the interaction term above revealed a significant Group × Reward Type interaction, multivariate F(1, 29) = 8.32, P < 0.008, but no significant main effects, P’s > 0.10. Follow-up within-groups t-tests revealed a significantly greater response to money than to object image rewards in the control group, P < 0.02, but a significantly greater response to object image rewards than to money in the ASD group, P < 0.05. Between-groups t-tests revealed significantly less response to money in the ASD relative to the control group, P < 0.005, and significantly greater response to objects in the ASD relative to the control group, P < 0.008. These results suggest that in the VMPFC, difference between groups with respect to anticipatory responses to rewards was contingent on reward type; however, this interaction term was driven by both greater activity in controls to money than objects, but the opposite pattern in the ASD group (i.e., greater activity to objects than money).
Fig. 5.
Brain areas showing significant Group × Reward Type interaction effects during outcomes and a bar graph depicting Z-score intensity values in the VMPFC cluster identified by significant interaction effects. Error bars represent standard errors of the mean.
Brain-symptom correlations
Correlations were evaluated between symptom severity scores (i.e. total and subscale scores of the RBS-R, the AQ and ADOS algorithms) and in-scanner RTs from participants in the ASD group with signal intensities within the right and left NAc clusters that predicted Group × Reward Type interactions during the anticipatory phase and within the VMPFC cluster that predicted Group × Reward Type interactions during the outcome phase. No significant relations were found.
DISCUSSION
Despite the potential relevance of reward system dysfunction to core autism symptoms, no studies to date have assessed neural responses during reward anticipation and outcomes in ASD. We employed a monetary incentive delay task that has been extensively used in the nonclinical literature (Knutson and Greer, 2008) and in multiple contexts in the clinical literature (Juckel et al., 2006; Wrase et al., 2007; Knutson et al., 2008; Strohle et al., 2008). In non-clinical contexts, the anticipation of monetary incentives recruits activation in NAc, as well as other basal ganglia structures, including the thalamus, putamen and caudate, whereas monetary outcomes recruit activation in medial and VMPFC, as well as basal ganglia structures, including the NAC, amygdala, caudate and putamen (Delgado et al., 2000; Knutson and Greer, 2008; Wacker et al., 2009; Pizzagalli et al., 2009; Forbes et al., 2009).
Responses during the monetary portion of the fMRI task in the present study revealed that the ASD group was characterized by relative NAc hypoactivation during monetary anticipation and outcomes, suggesting reward system dysfunction in ASDs in response to monetary incentives. Responses to object incentives, conceptually derived from reports of stimuli that are the focus of restricted interests in ASDs (e.g. trains, electronic devices; South et al., 2005; Klin et al., 2007) and that capture visual attention in ASDs (Sasson et al., 2008, 2010), revealed a different pattern of data. The ASD group was not characterized by decreased reward circuitry activation during either anticipatory or outcome phases of the task in response to object incentives. Rather, the dorsal anterior cingulate cortex, a region more typically associated with deficits in cognitive control in ASDs (Dichter et al., 2009a), showed decreased activation in the ASD group during object image anticipation, whereas the VMPFC showed relatively greater activation in the ASD group.
To more fully understand the pattern of responses to both types of rewards, Group × Reward Type interactions were evaluated for both temporal phases of the MID task. This approach yielded a more focused and constrained pattern of results. During anticipation, groups differed in responses to the two types of rewards in bilateral NAc and the left-caudate nucleus, reflecting that the ASD group was characterized by blunted anticipatory responses to both types of rewards relative to response of control participants to monetary incentives. During the outcome phase of the task, groups differed in responses to the two types of rewards in a VMPFC cluster, reflecting relative VMPFC hyperactivation to object incentives, relative to money incentives, in the ASD group.
Localization of differential anticipatory activation to the NAc and caudate implicates core brain regions mediating reward-based motivational salience (Berridge, 1996). The caudate nucleus mediates neurobiological processes that serve to link rewards to behavior, reward-related decision-making and encoding motivational feedback (Balleine et al., 2007; Lau and Glimcher, 2007). Blunted striatal anticipatory reward responses have been observed in other psychiatric disorders, such as major depressive disorder (Smoski et al., 2009) and schizophrenia (Juckel et al., 2006), suggesting potential shared neurobiology between ASD and other disorders with regards to reward-based anticipatory processes.
Dawson et al. (2004, 2005) hypothesized that ASDs are characterized by a social motivation deficit such that social information is not rewarding and thus does not serve to guide and shape social behavior. The present results suggest that social motivation deficits may reflect blunted anticipatory response to rewards more broadly. We thus place this pattern of findings within a broader Social Cognitive and Affective Neuroscience perspective of ASDs, suggesting that the disorder may be categorized within a broader framework of dysregulated motivational processes. Though the present study did not measure responses to social stimuli per se, the pattern of findings is consistent with an anticipatory pleasure deficit in response to a classic laboratory reward in individuals with ASDs.
The patterns of responses during the outcome phase of the task suggest relative hyperactivation in response to object incentives in ASDs. The VMPFC has been implicated in processing monetary outcomes in nonclinical samples (Knutson et al., 2000, 2001, 2003), and the NAc, a region implicated in reward outcomes as well (Delgado et al., 2000; Forbes et al., 2009). This finding is consistent with a model that circumscribed interests in ASDs may reflect hyperreactive reward brain circuitry in response to certain classes of stimuli and helps explain the circumscribed interests that are highly prevalent in autism (Klin et al., 2007; Lam et al., 2008) and clinical reports of the intense sense of pleasure reported by individuals with ASD about their circumscribed interests (Mercier et al., 2000). In this regard, the VMPFC cluster that showed ASD hyperactivity in response to object incentives was localized to the paracingulate gyrus, a region that mediates representations of others’ mental states (Gobbini et al., 2007) and that has shown decreased synchrony in autism during mentalizing tasks (Kana et al., 2009).
RT data revealed that the neurotypical group was faster at responding during the imaging task, a pattern that is consistent with reports of non-specific RT delays in individuals with ASDs in a variety of contexts (South et al., 2008). Both groups responded more quickly on trails with potential monetary rewards relative to potential image rewards, but there was no differential effect of diagnostic status on RTs to both trial types. Thus, fMRI patterns did not recapitulate behavioral responses, highlighting the unique information conveyed by the functional neuroimaging data.
Correlations between activation magnitudes in a priori regions of interests, in-scanner behavioral responses and severity of autism symptoms were not significant. Power to detect correlations was likely attenuated by the low scores on measure of ASD symptoms in this high-functioning sample, particularly the AQ, on which a substantial number of ASD participants did not score above the recommended autism cutoff (Baron-Cohen et al., 2001). Additionally, participants with ASDs were not selected based on the presence of high levels of RRBs or circumscribed interests in light of the diagnostic criteria for high-functioning autism (American Psychiatric Association, 1994).
Although the monetary condition in the present study was included as a standard laboratory reward that is ubiquitously used as a proxy for primary rewards, we acknowledge that there may be social influences on perceived monetary value. In other words, the value of money may be contingent on an exchange with another person, and thus the social deficits that are a defining feature of autism may influence neural responses to money. Additionally, because participants were adults, it is not possible to discern the extent to which altered responses to social and object rewards may have influenced the maturation of experience-dependent and experience-expectant portions of reward circuits during development (Meredith et al., 2008). Future pediatric studies of reward system function in autism will be necessary to more fully understand the effects of reward system function on the emergence of autism symptoms.1
Interpretation of brain-activation patterns to salient objects in the present study warrants caution for a number of reasons. First, a standard set of object images rather than images of participant-specific interests was used to allow for a direct comparison of responses between participants with and without ASDs and to allow for follow-up and replication studies using the same stimulus set. In this regard, the object image set was used not as a proxy for person-specific interests but rather as a ‘press’ to recruit brain reward networks in the context of the fMRI task. Similar approaches have been used to investigate not only other areas of deficits in ASDs, such as responses to standard face stimuli (Pelphrey et al., 2002; Corbett et al., 2009), but also to investigate a host of deficits in other forms of psychopathology, including investigations of emotional processing in schizophrenia (Fahim et al., 2004), unipolar depression (Dichter et al., 2009b, c), mania (Bermpohl et al., 2009) and posttraumatic stress disorder (Morey et al., 2008). The use of a standardized object image set likely provides a conservative estimate of the effects that may be observed with person-specific interests, and a next logical step in this line of research would be to extend this paradigm to include person-specific interests.
Additionally, although we cannot definitely conclude that viewing these object images was rewarding for individuals with ASDs, the eyetracking validation studies that were the basis for selecting these images indicated relatively greater visual saliency of these images in individuals with ASDs (Sasson et al., 2008, 2010). These eye-tracking results, combined with non-clinical brain imaging data suggesting inherent linkages between striatal activation magnitude, stimulus reward value, and stimulus saliency (Zink et al., 2004, 2006), suggest that these images were coded as having reward value in individuals with ASDs. We also note that the Sasson and colleagues (2008, 2010) eye-tracking validation studies were based on data from children, whereas the present sample was comprised of adults. Future eyetracking studies will be necessary to establish that adults with ASDs demonstrate similar patterns of visual attention to these stimuli. In this regard, however, we note that lifespan developmental studies have shown that the severity of repetitive behaviors can maintain with advancing age (Piven et al., 1996; Esbensen et al., 2008).
Another interpretive caution relates to the use of the WASI as an estimate of intelligence. This shortened form of a full intelligence test such as the WAIS (Wechsler, 1981) may accentuate the effects of the peaks and troughs known to occur on specific subtests in individuals with ASDs (Lockyer and Rutter, 1970). Additionally, although brain-imaging results were nearly identical when performance IQ was entered as a covariate, a group of control participants well matched on intelligence would improve the internal validity of findings (Mervis and Klein-Tasman, 2004). Finally, we did not assess for comorbid psychiatric conditions in our ASD sample, a design feature of future studies that will be necessary to conclusively link the patterns of brain imaging responses to symptoms of autism per se. This is particularly noteworthy given the high rates of comorbid psychiatric disorders in ASDs that are also characterized by reward system dysfunction (Leyfer et al., 2006; Cederlund et al., 2010).
The present data suggest a possible neurobiological mechanism for the social deficits and restricted interests that are commonly reported in ASDs (American Psychiatric Association, 1994; Klin et al., 2007; Lam et al., 2008). Aberrant functioning of reward pathways to standard laboratory rewards is a novel conceptualization of the pathophysiology of ASDs. Much is known about the role of dysregulated reward circuitry in other neuropsychiatric disorders (Kalivas and Volkow, 2005; Juckel et al., 2006; Kienast and Heinz, 2006; Dunlop and Nemeroff, 2007; Kirsch et al., 2007; Dichter et al., 2009b, c), and we speculate that reward-circuitry dysfunction in various neuropsychiatric disorders may be contingent on different classes of rewarding stimuli, a speculation that may account for the disparate symptom profiles across disorders that share this pathophysiologic trait. An increased understanding of reward processes in these disorders has provided a direction for the development of both psychosocial and psychopharmacological treatments (Nestler and Carlezon, 2006; Petry et al., 2005; Roll et al., 2004). If reward circuitry dysfunction plays a causal role in the development and expression of autism symptoms, then reward processes and their underlying neural circuitry may provide reasonable targets for the development of novel therapeutic approaches in autism.
Acknowledgments
Assistance for this study was provided by the Subject Registry and Neuroimaging Cores of the UNC Neurodevelopmental Disorders Research Center (P30 HD03110). The authors thank Dr Brian Knutson for kindly sharing the MID task, Josh Bizzell, Chris Petty and Todd Harshbarger for assistance with image analysis and MRI technologists Susan Music, Natalie Goutkin and Luke Poole for assistance with data acquisition. Finally, we thank two anonymous reviewers for insightful comments on an earlier draft of this article. This research was supported by the National Institute of Mental Health (K23MH081285 to G.S.D. and R01MH073402 to J.W.B.), by grants from the Dana Foundation and the Foundation of Hope to (G.S.D.).
APPENDIX 1
Fig. A1.
Object images.
Footnotes
1 We thank an anonymous reviewer for raising these points.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th edn. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. Journal of Neuroscience. 2007;27(31):8161–5. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Dalanay U, Kahnt T, et al. A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorder. 2009;11(1):70–75. doi: 10.1111/j.1399-5618.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Food reward: brain substrates of wanting and liking. Neuroscience Biobehavioural Review. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Bodfish JW, Symons FW, Lewis MH. The Repetitive Behavior Scale-Revised. Western Carolina Center Research Reports. 1999 [Google Scholar]
- Canli T, Zhao Z, Desmond JE, Kang E, Gross J, Gabrieli JD. An fMRI study of personality influences on brain reactivity to emotional stimuli. Behavioural Neuroscience. 2001;115(1):33–42. doi: 10.1037/0735-7044.115.1.33. [DOI] [PubMed] [Google Scholar]
- Cederlund M, Hagberg B, Gillberg C. Asperger syndrome in adolescent and young adult males. Interview, self- and parent assessment of social, emotional, and cognitive problems. Res Dev Disabil. 2010;31(2):287–98. doi: 10.1016/j.ridd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Carmean V, Ravizza S, et al. A functional and structural study of emotion and face processing in children with autism. Psychiatry Research. 2009;173(3):196–205. doi: 10.1016/j.pscychresns.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A. Defining the early social attention impairments in autism: social orienting, joint attention, and responses to emotions. Developmental Psychology. 2004;40:271–83. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychology. 2005;27(3):403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Social Cognitive and Affective Neuroscience. 2009a;4(3):215–26. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry. 2009b;66(9):886–97. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Smoski MJ. Affective context interferes with cognitive control in unipolar depression: an fMRI investigation. Journal of Affect Disorder. 2009c;114(1–3):131–42. doi: 10.1016/j.jad.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64(3):327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, et al. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42(12):1585–97. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Esbensen AJ, Seltzer MM, Lam KS, Bodfish JW. Age-related differences in restricted repetitive behaviors in autism spectrum disorders. Journal of Autism Developmental Disorder. 2008;39(1):57–66. doi: 10.1007/s10803-008-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim C, Stip E, Mancini-Marie A, Boualem M, Malaspina D, Beauregard M. Negative socio-emotional resonance in schizophrenia: a functional magnetic resonance imaging hypothesis. Medical Hypotheses. 2004;63(3):467–75. doi: 10.1016/j.mehy.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Koralek AC, Bryan RE, Montgomery KJ, Haxby JV. Two takes on the social brain: a comparison of theory of mind tasks. Journal of Cognitive Neuroscience. 2007;19(11):1803–14. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Biological Sciences. 2007;274(1619):1751–6. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–52. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. 2006;5(1):109–31. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cereb Cortex. 2010;21(4):769–76. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients. Pharmacopsychiatry. 2007;40(5):196–98. doi: 10.1055/s-2007-984463. [DOI] [PubMed] [Google Scholar]
- Klin A, Danovitch JH, Merz AB, Volkmar F. Circumscribed interests in higher functioning individuals with austim spectrum disorders: an exploratory study. Research and Practice for Persons with Severe Disabilities. 2007;32(2):89–100. [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–92. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363(1511):3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–7. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The repetitive behavior scale-revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–66. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2008;49(11):1193–200. doi: 10.1111/j.1469-7610.2008.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau B, Glimcher PW. Action and outcome encoding in the primate caudate nucleus. Journal of Neurosciences. 2007;27(52):14502–14. doi: 10.1523/JNEUROSCI.3060-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of Autism and Developmental Disorders. 2006;36(7):849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockyer L, Rutter M. A five- to fifteen-year follow-up study of infantile psychosis. IV. Patterns of cognitive ability. The British journal of social and clinical psychology. 1970;9(2):152–63. doi: 10.1111/j.2044-8260.1970.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23. [PubMed] [Google Scholar]
- Mercier C, Mottron L, Belleville S. A psychosocial study on restricted interests in high functioning persons with pervasive developmental disorders. Autism. 2000;4(4):404–25. [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Structure and Function. 2008;213(1–2):17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP. Methodological issues in group-matching designs: alpha levels for control variable comparisons and measurement characteristics of control and target variables. Journal of Autism and Developmental Disorders. 2004;34(1):7–17. doi: 10.1023/b:jadd.0000018069.69562.b8. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Research. 2008;162(1):59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Neal R. Neural plasticity, joint attention and a transactional social-orienting model of autism. In: Glidden L, editor. International Review of Research in Mental Retardation. Vol. 23. New York: Academic Press; 2001. pp. 139–68. [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biolgical Psychiatry. 2006;59(12):1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–61. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Archives of General Psychiatry. 2005;62(10):1148–56. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- Piven J, Harper J, Palmer P, Arndt S. Course of behavioral change in autism: a retrospective study of high-IQ adolescents and adults. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(4):523–9. doi: 10.1097/00004583-199604000-00019. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, et al. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166(6):702–10. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat P, Striano T. Early social cognition: understanding others in the first months of life. In: Rochat P, editor. Social-cognitive Development in the First Year. Mahwah, NJ: Erlbaum; 1999. pp. 3–34. [Google Scholar]
- Roid GH, Miller LJ. Leiter International Performance Scale-revised. Stoelting Co: Woodale, IL; 1997. [Google Scholar]
- Roll JM, Chermack ST, Chudzynski JE. Investigating the use of contingency management in the treatment of cocaine abuse among individuals with schizophrenia: a feasibility study. Psychiatry Research. 2004;125(1):61–4. doi: 10.1016/j.psychres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Sasson N, Turner-Brown L, Holtzclaw T, Lam KS, Bodfish J. Children with autism demonstrate circumscribed attention during passive viewing of complex social and nonsocial picture arrays. Autism Research. 2008;1:31–42. doi: 10.1002/aur.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, Bodfish JW. Brief Report: Circumscribed Attention in Young Children with Autism. Journal of Autism and Developmental Disorders. 2010 doi: 10.1007/s10803-010-1038-3. [10 May 2005; epub ahead print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG. Neural correlates of reward in autism. British Journal of Psychiatry. 2008;192:19–24. doi: 10.1192/bjp.bp.107.036921. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, et al. fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders. 2009;118:69–78. doi: 10.1016/j.jad.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive behavior profiles in Asperger syndrome and high-functioning autism. Journal of Autism and Developmental Disorders. 2005;35(2):145–58. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, Suchy Y, Kesner RP, McMahon WM, Lainhart JE. Intact emotion facilitation for nonsocial stimuli in autism: is amygdala impairment in autism specific for social information? Journal of the International Neuropsychological Society: JINS. 2008;14(1):42–54. doi: 10.1017/S1355617708080107. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50(4):1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohle A, Stoy M, Wrase J, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39(3):966–72. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Turner-Brown L, Lam K, Holtzclaw T, Dichter G, Bodfish J. Phenomenology and measurement of circumscribed interests in autism. Autism: International Journal of Research and Practise. 2011;15(4):437–56. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MA. Annotation: repetitive behaviour in autism: a review of psychological research. Journal of Child Psychology and Psychiatry. 1999;40(6):839–49. [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46(1):327–37. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- Weschler D. Weschler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14(6):1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–94. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29(3):977–83. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]