Abstract
Variability in gross tumour volume (GTV) definition is a major source of systematic error in conformal radiotherapy. This prospective study assesses the role of multidisciplinary collaboration between oncologists and radiologists in defining lung cancer volumes. Twenty patients with non–small cell lung cancer due to receive three-dimensional conformal radiotherapy formed the study population. GTVs were defined by a radiologist (GTVrad) and an oncologist (GTVonc) using available clinical information and imaging. A collaborative meeting was then held to agree on a final, common GTV (GTVfin) to be used for treatment planning, and differences analysed. The collaboration changed the GTV in 19/20 patients with a total of 50 regions being edited. Changes made were categorized as (a) differentiation of tumour from atelectasis or ground glass shadowing, (b) separation of tumour from vasculature, and (c) defining mediastinal extent of tumour. Oncologists were more confident in the GTVfin than the GTVonc. The radiologist took longer to define the GTV than the oncologist. Real-time collaborative GTV definition by a radiologist and oncologist is practical and feasible. This approach allows specific areas of uncertainty to be categorized and focussed on, reducing systematic error in GTV definition. The physician's approach to risk and decision making for each patient may also play a role.
Keywords: Gross tumour volume, lung cancer, radiologists, radiotherapy, systematic errors
Introduction
Computed tomography (CT) planned three-dimensional (3D) conformal radiotherapy (CRT) has been established as the standard of care for the non-surgical curative treatment of non–small cell lung cancer[1,2]. Defining volumes as set out in International Commission on Radiation Units (ICRU) reports 50 and 62 is central to radiotherapy planning[3,4]. The first step is delineation of the gross tumour volume (GTV), which is “the gross demonstrable extent and location of tumour growth, defined by clinical examination and/or imaging”. It is well established in lung and other cancers that there is considerable inter- and intra-observer variability in GTV definition[5–12]. Some of this variability reflects the genuine unknown nature of the location of the GTV. This can only accurately be defined by clinicopathologic correlation on an individual basis, which is impractical[13]. However, the magnitude of the total variability suggests that a more formal approach to GTV definition could lead to greater agreement between physicians and a reduction in the systematic error that errors in volume delineation create[14]. This systematic error is magnified as the GTV is expanded to form a clinical target volume (CTV) and planning target volume (PTV). Refining the way the GTV is defined is thus critical.
Volume definition is usually carried out by radiation oncologists, sometimes with the help of radiologists to interpret uncertainties in the imaging. Retrospective studies show that radiologists working independently usually draw smaller GTVs than oncologists in lung cancer[6]. The UK Royal College of Radiologists has produced guidance on how oncologists and radiologists can work together to define the GTV: “It is recommended that the clinical oncologist and clinical radiologist work together to define the GTV for specific tumour types. Once appropriately trained, the clinical oncologist should be able to undertake GTV definition for routine work such as prostate and lung cases. The clinical oncologist and clinical radiologist should meet on a regular basis (at least weekly) to discuss and jointly plan more complex cases such as head and neck cancers and sarcoma.”[15]
A more systematic approach to GTV definition is also being developed by a Royal College of Radiologists (UK) working party as outlined in a recent paper[16].
The purpose of this study was to assess and develop a useful model of collaborative volume definition. This multidisciplinary approach to GTV definition was first investigated in a retrospective study in several tumour types in order to develop useful methods for recording the volumes and the effects of the collaboration[17]. The radiologist–oncologist collaboration was particularly helpful in defining lung cancer volumes. Here we report the results of the first study to look at the impact of a radiologist helping the oncologist to define the GTV prospectively in patients having conformal radiotherapy.
Materials and methods
Twenty patients having 3D conformal CT-planned radiotherapy for non–small cell lung cancer formed the study population. Patient and treatment characteristics are listed in Table 1. The patients were treated with radiotherapy alone or with sequential chemotherapy and radiation.
Table 1.
Patient and treatment characteristics
| Characteristics | No. of patients | |
|---|---|---|
| Age (years) | Average | 72 |
| Range | 35–85 | |
| Sex | Male | 13 |
| Male | Female | 7 |
| Stage | Ia | 1 |
| Ib | 3 | |
| IIa | 0 | |
| IIb | 1 | |
| IIIa | 8 | |
| IIIb | 6 | |
| IV | 1 | |
| Radiotherapy | 55 Gy/20# | 14 |
| 39 Gy/13# | 1 | |
| 30 Gy/6# | 3 | |
| 27 Gy/6# | 2 | |
| Chemotherapy | Gemcitabine/carboplatin | 10 |
| Vinorelbine/carboplatin | 4 | |
| No chemotherapy | 6 | |
| Average no. cycles | 3 | |
| Range | 1–6 | |
All patients had had an intravenous contrast-enhanced diagnostic CT scan as part of their initial lung cancer staging investigations. The study immediately predated availability of positron emission tomography (PET)/CT in our institution. Planning images were obtained on a GE high-speed single-slice spiral CT scanner in the treatment position with the patient supine, arms above the head, and immobilized with a customized T-bar device. Images were acquired with the patient free breathing with a 3-mm slice thickness reconstructed every 3 mm. Each set of images was acquired over approximately 30 s without intravenous contrast, as per standard departmental protocol. Images were transferred digitally to the Eclipse radiotherapy planning system.
One consultant thoracic radiologist and one of 2 consultant thoracic radiation oncologists defined a GTV for each patient independently (GTVrad and GTVonc, respectively). For each patient the physician was given access to the electronic patient record. All radiologic investigations were available to the physicians electronically via PACS (Picture Archiving and Communications System) with screens adjacent to the radiotherapy planning computer.
Each doctor was asked to define the visible extent of the tumour and any involved lymph nodes. We considered nodes ≥10 mm in short axis diameter to be involved by tumour. In patients who had received neoadjuvant chemotherapy, the GTV included all initial sites of disease (e.g. lymph nodes larger than 10 mm on diagnostic CT that had been reduced to <10 mm by chemotherapy). Lung windowing (W=1600 and L=−600) was used to define the extent of pulmonary disease and soft tissue windowing (W=400 and L=20) was used to define lymph nodes and mediastinal tumour extent as described in the ESTRO literature-based recommendations for lung cancer radiotherapy[18]. Participants rated their degree of confidence in the GTV they had defined as representing the true tumour volume on a 10-point Likert scale (1, not at all confident; 10, extremely confident). The time taken to define the GTV was recorded as were any specific difficulties encountered.
The physicians then had a collaborative meeting to review the GTV on each CT slice. The oncologist's GTV was copied and modified by the oncologist during the meeting to produce a common, final GTV (GTVfin) to be used for treatment planning. Reasons for differences between the oncologist's (GTVonc) and the collaborative (GTVfin) volumes were recorded. Participants also rated their confidence in the collaborative GTV after the meeting, and the time taken for this meeting was noted.
This final GTV was subsequently used by the oncologist to produce a CTV (GTV + 8 mm) and then a PTV (CTV + 7 mm axially, 12 mm superiorly and inferiorly). This PTV was used as the basis for the treatment plan for each patient.
The 3 GTVs (GTVonc, GTVrad and GTVfin) were compared quantitatively in terms of the volume (cm3) and the concordance index (CI). This is defined as the ratio of volume of intersection (∩) of the GTVs to the volume of union (∪) of the GTVs[6]. When comparing GTVonc and GTVfin, the CI is thus defined as:
This can therefore range between 0 (complete disagreement between the 2 volumes) and 100 (perfect agreement). This method is sensitive for small variations in overlap as well as different volume sizes.
Results
Time
The radiologist took longer to define a GTV than the oncologist (mean 26.2 vs 17.4 min, P<0.005, 2-tailed paired t-test). The mean time for the joint meeting to agree on a final, collaborative GTV to be used for treatment planning was 15 min (range 5–30 min). There was no evidence that GTV definition became faster as the study progressed.
Confidence in volumes
The radiologist was more confident that his GTV was correct (mean 7.7, range 6–10) than the oncologist (mean 6.1, range 4–8) P<0.0005, 2-tailed paired t-test. The collaborative meeting led to an increase in the oncologist's confidence in the GTV in all patients, by a mean of 2.5 points, range 1–4. However the radiologist did not feel more confident after the collaborative meeting. This suggests that the oncologists found the radiologist's input beneficial in improving GTV delineation.
Volume sizes and concordance
The GTVonc (mean 74.0 cm3, range 13.3–295.0 cm3) was not significantly larger than the GTVrad (mean 81.0 cm3, range 8.8–297.9 cm3), P=0.15, 2-tailed paired t-test.
The collaborative meeting produced a different GTVfin from the oncologist's GTVonc in 19 of the 20 patients studied. The GTVfin was larger than the GTVonc in 16 cases (increased by mean of 11.4%) and smaller in 3 (decreased by mean of 8.4%). The CI between the GTVonc and GTVfin varied from 40 to 100 with a mean of 80. The CI was greater than 90 in 3 out of the 4 patients with stage I disease, which were more straightforward to delineate due to the lack of invasion of other structures. However, 1 of the 4 patients had a below average CI of 73, and required different areas of atelectasis/tumour to be removed or added, and part of a vessel removed from the GTVfin.
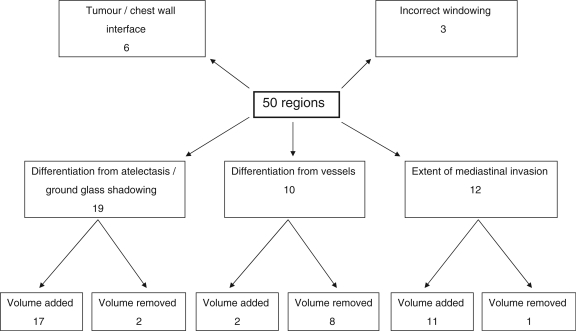
In the 20 patients there were a total of 50 regions (range 0–6 per patient) where the GTVonc was altered at the planning meeting in the production of the GTVfin. In 3 of the patients the physicians felt that PET/CT imaging would have improved the accuracy of GTV definition. The changes made to the regions are summarized below and shown schematically in Fig. 1. Examples of different types of changes are illustrated in Figs. 2–4.
19 regions altered due to differentiation of tumour from atelectasis or ground glass shadowing
12 regions altered due to change of extent of mediastinal invasion
10 regions altered due to differentiation from vasculature
6 regions altered at the tumour/chest wall interface
3 regions altered due to incorrect window levels
Figure 1.
Classification of changes made to GTVonc during collaborative meeting to produce GTVfin.
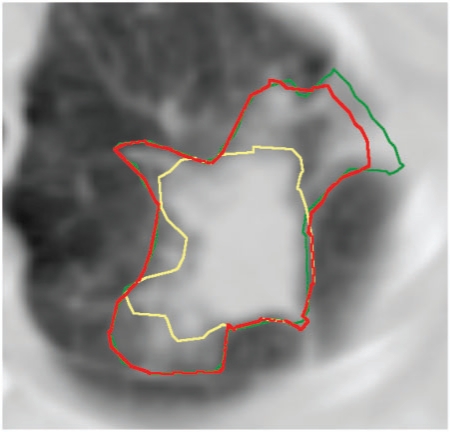
Figure 2.
Differentiation between tumour and tumour-related non-malignant change. The GTVfin is larger than the oncologist's GTV as a larger area of ground glass change has been included after discussion with the radiologist. GTVonc, yellow; GTVrad, green; GTVfin, red.
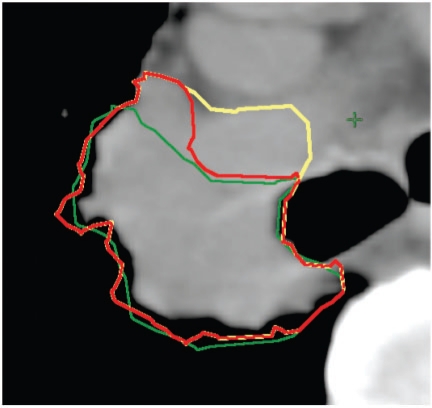
Figure 3.
Differentiation between tumour and vasculature. The extent of invasion of the tumour into the right pulmonary artery is difficult to assess and there is also a partial volume effect, so the exact position of the boundary is not known. In this example, a compromise between the different outlines was reached to produce GTVfin. GTVonc, yellow; GTVrad, green; GTVfin, red.
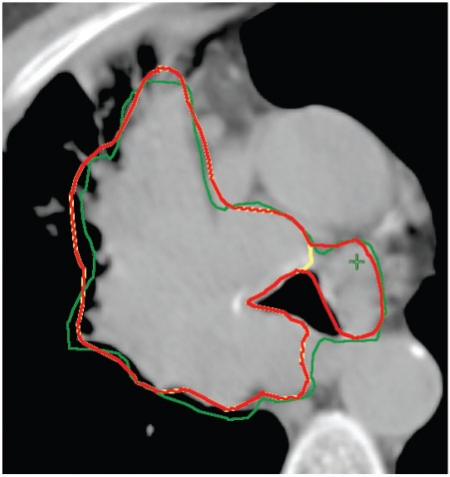
Figure 4.
Assessing the mediastinal extent of disease. The oncologist's GTV is extended to include more mediastinal extent of tumour, in this case a previously unrecognised aortopulmonary lymph node. GTVonc, yellow; GTVrad, green; GTVfin, red.
Discussion
Accurate target volume definition in CRT has the potential to improve the therapeutic ratio by allowing dose escalation, which may subsequently lead to increased cure rates[19]. Definition of the GTV on a planning CT scan is the first step in achieving this. Previous research has shown that different physicians interpret GTVs differently and that oncologists and radiologists define different volumes[5–8,20]. Better agreement between physicians defining GTVs in lung cancer can be achieved if 3 areas of uncertainty are focussed on:
Differentiation of tumour from atelectasis/ground glass shadowing
This was the most common type of uncertainty in this study. In most cases, the GTVfin included more areas of atelectasis or ground glass shadowing. This has the effect of improving the likelihood of tumour cell kill if these areas include malignant disease, but if these areas are benign, it increases the amount of normal tissue included and thus worsens the therapeutic ratio. In this study the GTV was defined using incorrect settings in 3 cases. Specifying window width and level in protocols and encouraging manufacturers to allow presetting of these in planning software will reduce this systematic error.
PET imaging alone or in conjunction with radiotherapy planning CT scans is helpful in distinguishing tumour from atelectasis, in lymph node staging, and in excluding distant metastatic disease[21]. Several series have shown that the use of PET in radiotherapy planning results in a change in treatment intent or target volume, usually by reducing the size of the GTV[21–26]. There are many different methods of using PET information to aid contouring the GTV, including visual interpretation, maximum standardized uptake value (SUVmax) of 2.5, or thresholding of a percentage of SUVmax[27]. No one method is suitable for all clinical scenarios, and the limitations of each technique should be weighed up[28]. Problems of respiratory motion, spatial resolution and immobilization may be partially overcome by the use of PET/CT and fusing the images to the radiotherapy planning CT, or by the use of a dedicated PET/CT simulator. In one series, radiotherapy planning using the latter in comparison with conventional CT planning has allowed dose escalation by reducing the radiation exposure to the dose-limiting organs of lung and oesophagus, potentially increasing the tumour control probability (TCP) from 6.3±1.5% to 24.0±5.6%[22].
Separation of tumour from vasculature
This type of uncertainty usually led to a reduction in the size of GTVfin as the oncologist had mistakenly included blood vessels in the GTV. Using the GTVfin to produce the PTV would therefore lead to an improvement in the therapeutic ratio by reducing the radiation dose to normal mediastinal structures, in particular the oesophagus.
Although using intravenous contrast for the planning CT may improve the accuracy of vessel definition, all patients had a recent contrast-enhanced diagnostic CT scan available on an adjoining computer in the planning room. The extra benefit of a contrast-enhanced planning CT scan can be inferred but has never been prospectively studied.
UK oncologists currently receive no formal training in cross-sectional anatomy. Identification of normal vessels and other mediastinal structures could be improved by incorporating formal training in CT-based anatomy into training programmes, or by using online atlases similar to the head and neck consensus guidelines for nodal volumes[29,30]. Combined training of oncologists and radiologists in oncologic cross-sectional anatomy would be useful to bring their 2 perspectives together.
Definition of the mediastinal extent of the tumour
For this category of uncertainty, PET scans, intravenous contrast and training in cross-sectional anatomy will again help to define the extent of both mediastinal invasion and nodal extent. But in most of the patients in this study, the physicians did not feel that further imaging would have been useful; the mediastinal tumour extent may be a genuine unknown. Collaborative volume definition in real time during planning by an oncologist and radiologist may therefore be the best way to bring together the 2 opinions and better define areas of uncertainty.
The study asked the oncologist and radiologist to define the GTV separately and then together. The radiologist took longer to do this – perhaps because of unfamiliarity with the planning software and the concepts of volume delineation although it is difficult to draw many conclusions as only one radiologist participated in the study. Nonetheless the additional time that a collaborative meeting took was manageable.
How physicians factor in the natural history of the cancer in an individual patient also needs to be considered in relation to the above issues. The radiation oncologist has a wide experience of treating patients with radical radiotherapy and therefore has a repertoire of knowledge of tolerable treatment volumes, the ability of patients to cope with treatments, and follow-up data in particular with respect to late toxicity and patterns of relapse. He has also met the specific patient being planned for treatment, and assessed their suitability and fitness for therapy. For example, a cautious approach may be taken in a patient with poor lung function on the basis that if all areas of uncertainty were included, the resulting pneumonitis would be intolerable. Conversely for a patient with excellent performance status and an otherwise long life expectancy where radiation is the only chance of long-term cure, the oncologist may wish to include all areas of uncertainty; the fear of a geographic miss outweighing the risk of side effects. This accepted uncertainty may explain why the oncologist's confidence increased after a collaborative meeting. The final GTV may still be modified on an individual patient and individual physician basis, despite the above areas of uncertainty being identified and potentially resolved. In contrast, the radiologist takes a more impartial view and contours the GTV without reference to individual patient characteristics so his confidence in the accuracy of the GTV does not change with the collaborative meeting.
The individual physician's approach to risk will also play a role; the more risk averse with respect to tumour recurrence will attempt to cover all possible sites of disease, while the less risk averse with respect to recurrence (or more risk averse for complications) will try to keep the volume small. The science of judgment and decision making as applied to medicine has revealed many biases in the way we assess risk in this kind of situation[31]. Value-induced bias infers that a probability estimate is distorted by allowing the undesirability of a possible outcome to alter the estimate of its likelihood of occurrence. The availability bias or heuristic states that the probability of recent and salient or rare but vivid events is overestimated and the probability of remote, less memorable or common, ordinary events is underestimated. So we may fear a marginal recurrence or a fatal pneumonitis more if we have recently encountered one in clinical practice – and (sub-)consciously adjust the GTV we define accordingly.
Other cognitive biases (e.g. ego bias, which suggests that physicians tend to overestimate our own performance and underestimate the performance of others[32]) also have implications for a collaborative approach to complex tasks. More research into the how the biases of judgment and decision making affect GTV definition may help to define strategies to improve concordance.
Defining the GTV is only one step in the chain of tasks required for successful radiotherapy treatment. Evidence-based GTV-CTV and CTV-PTV expansion are also important in producing a plan to treat the tumour and minimize normal tissue toxicity[14]. If the margins from CTV to PTV are larger than necessary, refining the GTV may have less effect on the therapeutic ratio than reducing the systematic and random errors in PTV definition, for example, by accounting for tumour motion[33]. The chain of tasks must be considered in its entirety so that the weakest link is improved as a priority.
The major limitation of this study and indeed all other work in defining the correct GTV is the lack of a gold standard. The few studies that have attempted to correlate CT volumes with pathologic specimens have been biased, for example, by the shrinkage of pathologic specimens and the problem of deflation of excised lung[34]. Careful long-term follow-up of all patients having 3D-CRT with the matching of relapse data to planning CT images is perhaps the best way to refine the accuracy of volume definition[35]. This study assumes that concordance between physicians in defining the GTV will produce a more accurate volume. If each physician has made the same error then the GTV may still be incorrect, but the different perspectives of experienced oncologists and radiologists should lead to greater accuracy. The study only investigated the approach of 1 radiologist and 2 oncologists specializing in the field of lung cancer and should be repeated with other physicians to ensure the data are reproducible.
Conclusions
Radiologists and oncologists can work together to define GTVs in lung cancer patients and produce volumes that are concordant and reflect their complementary expertise and perspectives. The most common areas of uncertainty and discussion include differentiation of tumour from tumour-related non-malignant change or vasculature, and defining the mediastinal extent of invasion. Newer techniques such as PET/CT may improve accuracy but some collaboration is still likely to be useful for many patients, particularly when defining the extent of primary tumour invasion of the mediastinum. Although this method may be time consuming initially, it can be expected to become faster over time as each specialist learns from the other. Such a collaborative approach will increase the confidence of the oncologist that the GTV is correct and will reduce the chance of a systematic error in GTV definition that will be magnified as the GTV is expanded to a PTV.
Acknowledgements
The authors would like to thank Penny Smith and Jenny Tomes from the radiotherapy department of Norfolk and Norwich Hospital for their technical assistance in planning.
Conflict of interest
None declared
Footnotes
This paper is available online at http://www.cancerimaging.org. In the event of a change in the URL address, please use the DOI provided to locate the paper.
References
- 1.Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable) Cochrane Database Syst Rev. 2001;((1)):CD002935. doi: 10.1002/14651858.CD002935. [DOI] [PubMed] [Google Scholar]
- 2.The Royal College of Radiologists Clinical Oncology Information Network. Guidelines on the non-surgical management of lung cancer. Clin Oncol (R Coll Radiol) 1999;11:S1–53. [PubMed] [Google Scholar]
- 3.Measurements ICoRUa. ICRU Report 50. Prescribing, recording and reporting photon beam therapy. Bethesda, MD: ICRU; 1993. [Google Scholar]
- 4.Measurements ICoRUa. ICRU Report 62 (Supplement to ICRU Report 50). Prescribing, recording and reporting photon beam therapy. Bethesda, MD: ICRU; 1999. [Google Scholar]
- 5.Cazzaniga LF, Marinoni MA, Bossi A, et al. Interphysician variability in defining the planning target volume in the irradiation of prostate and seminal vesicles. Radiother Oncol. 1998;47:293–6. doi: 10.1016/S0167-8140(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 6.Giraud P, Elles S, Helfre S, et al. Conformal radiotherapy for lung cancer: different delineation of the gross tumor volume (GTV) by radiologists and radiation oncologists. Radiother Oncol. 2002;62:27–36. doi: 10.1016/S0167-8140(01)00444-3. [DOI] [PubMed] [Google Scholar]
- 7.Tai P, Van Dyk J, Yu E, Battista J, Stitt L, Coad T. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;42:277–88. doi: 10.1016/s0360-3016(98)00216-8. [DOI] [PubMed] [Google Scholar]
- 8.Van de Steene J, Linthout N, et al. Definition of gross tumor volume in lung cancer: inter-observer variability. Radiother Oncol. 2002;62:37–49. doi: 10.1016/S0167-8140(01)00453-4. [DOI] [PubMed] [Google Scholar]
- 9.Gao Z, Wilkins D, Eapen L, Morash C, Wassef Y, Gerig L. A study of prostate delineation referenced against a gold standard created from the visible human data. Radiother Oncol. 2007;85:239–46. doi: 10.1016/j.radonc.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Seddon B, Bidmead M, Wilson J, Khoo V, Dearnaley D. Target volume definition in conformal radiotherapy for prostate cancer: quality assurance in the MRC RT-01 trial. Radiother Oncol. 2000;56:73–83. doi: 10.1016/S0167-8140(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 11.Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;47:285–92. doi: 10.1016/S0167-8140(98)00021-8. [DOI] [PubMed] [Google Scholar]
- 12.McJury M, Dyker K, Nakielny R, Conway J, Robinson MH. Optimizing localization accuracy in head and neck, and brain radiotherapy. Br J Radiol. 2006;79:672–80. doi: 10.1259/bjr/14663755. [DOI] [PubMed] [Google Scholar]
- 13.Yu HM, Liu YF, Hou M, Liu J, Li XN, Yu JM. Evaluation of gross tumor size using CT, (18)F-FDG PET, integrated (18)F-FDG PET/CT and pathological analysis in non-small cell lung cancer. Eur J Radiol. 2008;72:104–13. doi: 10.1016/j.ejrad.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 14.van Herk M. Errors and margins in radiotherapy. Semin Radiat Oncol. 2004;14:52–64. doi: 10.1053/j.semradonc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Radiologists TRCo. Imaging for oncologists. London: Royal College of Radiologists; 2004. [Google Scholar]
- 16.Jefferies S, Taylor A, Reznek R. Results of a National Survey of Radiotherapy Planning and Delivery in the UK in 2007. Clin Oncol (R Coll Radiol) 2009;21:204–17. doi: 10.1016/j.clon.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Horan G, Roques TW, Curtin J, Barrett A. “Two are better than one”: a pilot study of how radiologist and oncologists can collaborate in target volume definition. Cancer Imaging. 2006;6:16–9. doi: 10.1102/1470-7330.2006.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senan S, De Ruysscher D, Giraud P, Mirimanoff R, Budach V. Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiother Oncol. 2004;71:139–46. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Belderbos JS, Heemsbergen WD, De Jaeger K, Baas P, Lebesque JV. Final results of a Phase I/II dose escalation trial in non-small-cell lung cancer using three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:126–34. doi: 10.1016/j.ijrobp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Roy AE, Wells P. Volume definition in radiotherapy planning for lung cancer: how the radiologist can help. Cancer Imaging. 2006;6:116–23. doi: 10.1102/1470-7330.2006.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashamalla H, Rafla S, Parikh K, et al. The contribution of integrated PET/CT to the evolving definition of treatment volumes in radiation treatment planning in lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1016–23. doi: 10.1016/j.ijrobp.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 22.De Ruysscher D, Wanders S, Minken A, et al. Effects of radiotherapy planning with a dedicated combined PET-CT-simulator of patients with non-small cell lung cancer on dose limiting normal tissues and radiation dose-escalation: a planning study. Radiother Oncol. 2005;77:5–10. doi: 10.1016/j.radonc.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 23.van Der Wel A, Nijsten S, Hochstenbag M, et al. Increased therapeutic ratio by 18FDG-PET CT planning in patients with clinical CT stage N2-N3M0 non-small-cell lung cancer: a modeling study. Int J Radiat Oncol Biol Phys. 2005;61:649–55. doi: 10.1016/j.ijrobp.2004.06.205. [DOI] [PubMed] [Google Scholar]
- 24.Deniaud-Alexandre E, Touboul E, Lerouge D, et al. Impact of computed tomography and (18)F-deoxyglucose coincidence detection emission tomography image fusion for optimization of conformal radiotherapy in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2005;63:1432–41. doi: 10.1016/j.ijrobp.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Lavrenkov K, Partridge M, Cook G, Brada M. Positron emission tomography for target volume definition in the treatment of non-small cell lung cancer. Radiother Oncol. 2005;77:1–4. doi: 10.1016/j.radonc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Steenbakkers RJ, Duppen JC, Fitton I, et al. Reduction of observer variation using matched CT-PET for lung cancer delineation: a three-dimensional analysis. Int J Radiat Oncol Biol Phys. 2006;64:435–48. doi: 10.1016/j.ijrobp.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Nestle U, Kremp S, Grosu AL. Practical integration of [18F]-FDG-PET and PET-CT in the planning of radiotherapy for non-small cell lung cancer (NSCLC): The technical basis, ICRU-target volumes, problems, perspectives. Radiother Oncol. 2006;81:209–25. doi: 10.1016/j.radonc.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 28.MacManus M, Nestle U, Rosenzweig KE, et al. Use of PET and PET/CT for radiation therapy: IAEA expert report 2006–2007. Radiother Oncol. 2009;91:85–94. doi: 10.1016/j.radonc.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 29.Gregoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol. 2006;79:15–20. doi: 10.1016/j.radonc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Gregoire V, Levendag P, Ang KK, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol. 2003;69:227–36. doi: 10.1016/j.radonc.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 31.Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185:1124–31. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 32.Detmer DE, Fryback DG, Gassner K. Heuristics and biases in medical decision-making. J Med Educ. 1978;53:682–3. doi: 10.1097/00001888-197808000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Panakis N, McNair HA, Christian JA, et al. Defining the margins in the radical radiotherapy of non-small cell lung cancer (NSCLC) with active breathing control (ABC) and the effect on physical lung parameters. Radiother Oncol. 2008;87:65–73. doi: 10.1016/j.radonc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys. 2000;48:1015–24. doi: 10.1016/S0360-3016(00)00750-1. [DOI] [PubMed] [Google Scholar]
- 35.Moreno-Jimenez M, Aristu J, Lopez-Picazo JM, et al. Dosimetric analysis of the patterns of local failure observed in patients with locally advanced non-small cell lung cancer treated with neoadjuvant chemotherapy and concurrent conformal (3D-CRT) chemoradiation. Radiother Oncol. 2008;88:342–50. doi: 10.1016/j.radonc.2008.05.019. [DOI] [PubMed] [Google Scholar]