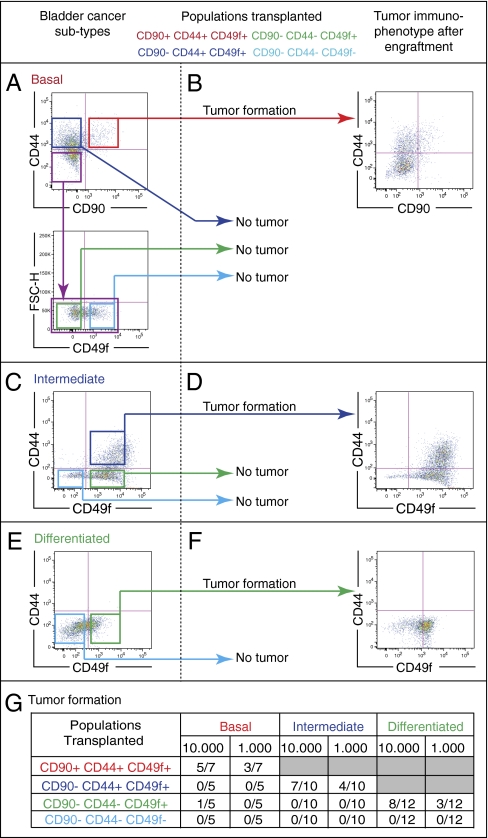
Fig. 4.
Functional validation of the computationally predicted differentiation states in BC. In vivo validation of three phenotypically distinct subtypes of bladder cancer (BC) according to their differentiation states: (A) basal, (C) intermediate, and (E) differentiated as defined by surface marker profiles (CD90, CD44, and CD49f). BC cells were purified by FACS and xenotransplanted intradermally into immunodeficient mice in limited dilution (103 and 104). (B, D, and F) The immunophenotypes of xenograft tumors derived from each BC subtype were reanalyzed by FACS postengraftment. (A) The basal BC subtype is composed of all differentiation states [CD90+CD44+CD49f+ (red box) → CD90−CD44+CD49f+ (blue box) → CD90−CD44−CD49f+ (green box) → CD90−CD44−CD49f− (light blue box)]. (B) Only the most upstream (CD90+CD44+CD49f+) population forms xenograft tumors and recapitulates all downstream differentiation states (CD90−CD44+CD49f+ → CD90−CD44−CD49f+ → CD90−CD44−CD49f−). (C) The intermediate BC subtype lacks the basal differentiation state (CD90+CD44+CD49f+). (D) Only the most upstream differentiation state (CD90−CD44+CD49f+) forms xenograft tumors and can reconstitute all downstream states (CD90−CD44−CD49f+ → CD90−CD44−CD49f−). (E) In differentiated BCs that lack both the basal (CD90+CD44+CD49f+) and the intermediate (CD90−CD44+CD49f+) differentiation states, (F) only the existing differentiation state (CD90−CD44−CD49f+) forms xenograft tumors and recapitulates the terminally differentiated (CD90−CD44−CD49f−) downstream state. (A, C, and E) The terminally differentiated subpopulations (CD90−CD44−CD49f−) never give rise to tumors. (G) Frequency of tumor formation of all transplanted cell populations described in A, C, and E.