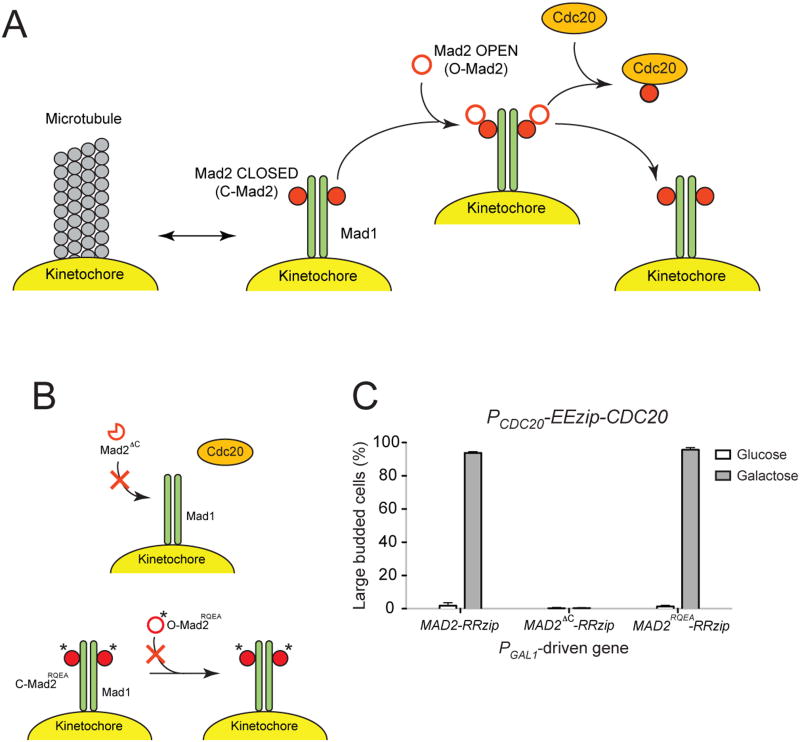
Figure 6.
. Metaphase arrest induced by tethering Mad2 mutants to Cdc20 supports the Mad2-template model. (A) The Mad2-template model (adapted from [6]). Mad1 dimers associate with unattached kinetochores and bind Mad2, converting them from “open” (O-Mad2) to “closed” (C-Mad2) conformation. The Mad1-Mad2 complexes at the kinetochores (the “templates”) then recruit additional open Mad2, allowing the formation of closed Mad2-Cdc20 complexes. (B) The behavior of Mad2 mutants in the context of the Mad2-template model. (Top) Mad2ΔC lacks the C-terminal amino acid residues and cannot convert to the closed Mad2 conformation. It fails to activate the spindle checkpoint since it is unable to form a stable complex with Mad1 and to bind to and inhibit Cdc20. (Bottom) The double point mutant Mad2RQEA carries the mutations Arg126-Glu and Gln127-Ala. The changes inhibit the binding between free Mad2 and closed conformation of Mad2 found in the Mad1-Mad2 complex, which inactivates the spindle checkpoint by preventing the formation of C-Mad2-Cdc20 complexes. The mutations also affect the interaction of Mad2 with BUBR1 (mammalian version of Mad3) and the formation of stable MCC. (C) Effects of tethering Mad2 mutants to Cdc20. Cells with PCDC20-EEzip-CDC20 and the indicated PGAL1-driven genes were released from G1 arrest into glucose- or galactose-containing media. The percentage of large budded cells was determined by light microscopy after 3 hours of growth. Error bars represent the standard deviation of three independent trials. Two hundred cells were counted for each trial. Tethering the Mad2 mutant that can reach the closed conformation, but cannot induced conformational conversion in other Mad2 molecules (Mad2RQEA), does activate the checkpoint, but tethering the mutant that cannot achieve the closed conformation (Mad2ΔC) does not.