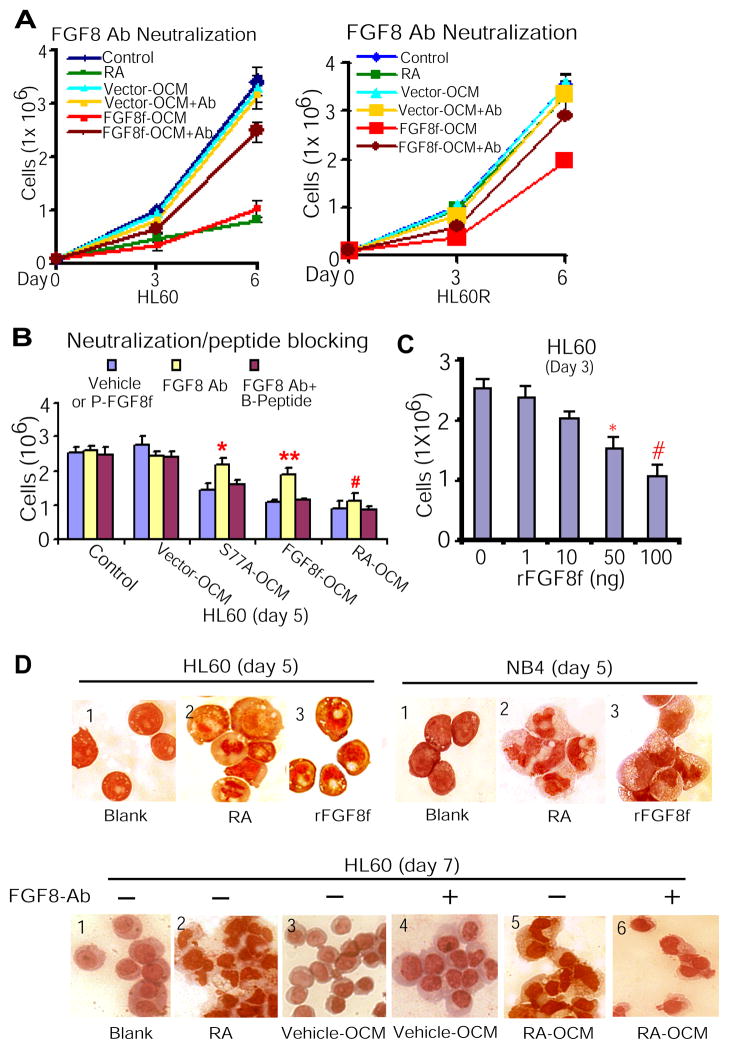
Figure 3. Paracrine FGF8f derived from osteoblastic niche cells is required for mediating granulocytic differentiation of myeloid leukemic cells.
(A) FGF8 antibodies (100 ng/ml) neutralized the inhibitory effect of FGF8f-OCM on HL60 (left) and HL60R cell proliferation (right). (B) The inhibitory effect of paracrine FGF8f in S77A-OCM or FGF8f-OCM or RA-OCM on HL60 cell proliferation (blue bar) was neutralized in the presence of FGF8f antibodies (yellow bar), whereas such neutralizing effect of FGF8f antibodies was reversed in the presence of blocking peptides (red bar). FGF8 Ab vs. FGF8 Ab + peptide: **P <0.002, *P<0.007, and #P<0.02. P-FGF8f, paracrine FGF8f that presents in S77A- or FGF8f- or RA-OCM; B-Peptide, blocking peptide for FGF8 antibodies. (C) HL60 cells treated with rFGF8f. P<0.0005; *P<0.002. (D) rFGF8f mimicked the effect of RA on inducing terminal granulocytic differentiation of HL60 and NB4 cells (top). The effect of RA-OCM containing FGF8f on inducing terminal granulocytic morphology differentiation was reversed by FGF8 antibodies (bottom).