Figure 6. Transthiolation in trans: a working model for transfer of Atg8 from Atg7 to Atg3.
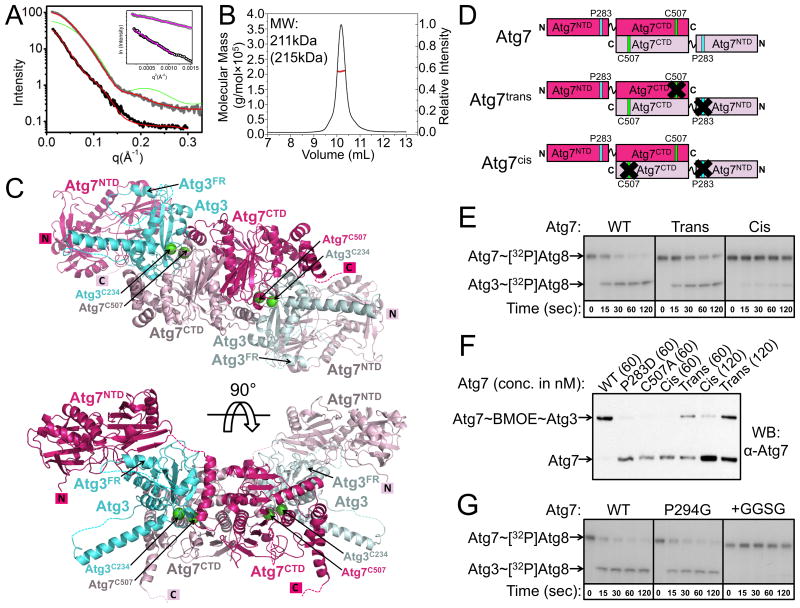
A) Analysis of the interference-free SAXS curve for Atg7CTD (gray) and Atg7 (black), with theoretical scattering calculated with FoXS (Schneidman-Duhovny et al., 2010) from crystal structure and atomistic model, respectively, in red (χ2 = 2.1, Atg7CTD; χ2 = 1.5, Atg7). For reference, a poorly fitting theoretical SAXS curve if Atg7CTD were a monomer is shown in green. Inset – Guinier plots indicating aggregation-free data (magenta).
B) SEC-MALS data plotted as a molar mass distribution (red) superimposed on chromatogram of differential refractive index as a function of elution volume. Shown is molecular weight (MW) determined by SEC-MALS, with theoretical value in parenthesis.
C) Model of homodimeric Atg7 (dark and light pink) bound to two Atg3s (cyan and light blue), with Atg7 and Atg3 catalytic cysteines (green spheres) in close proximity for catalysis. The Cys of the cyan Atg3 bound via its FR to the NTD of the dark pink Atg7 is close to the Cys in the CTD of the light pink Atg7, enabling transthiolation in trans. For clarity, Atg8, which would be transferred from the catalytic Cys of Atg7 to the catalytic Cys of Atg3, is not shown.
D) Schematics of wild-type Atg7, and Atg7trans and Atg7cis dimers. Pro283 that binds Atg3FR is cyan and Atg7's catalytic Cys507 is green. Black Xs indicate mutation of Pro283 to Asp or Cys507 to Ala to impair binding of Atg3 or prevent thiolester formation with Atg8, respectively.
E) Autoradiogram for time course of transfer of [32P]Atg8 from Atg7 to Atg3. WT - wild-type Atg7 homodimer; trans - Atg7 heterodimer composed of Atg7(P283D) and Atg7(C507A); cis - Atg7 heterodimer composed of wild-type Atg7 and Atg7(P283D, C507A).
F) BMOE-crosslinking between Atg3C234only and the indicated variants of Atg7 at concentrations of either 60 or 120 nM, detected by anti-Atg7 western.
G) Autoradiogram showing time course of [32P]Atg8 transfer to Atg3 from the indicated versions of Atg7.