FIG. 9.
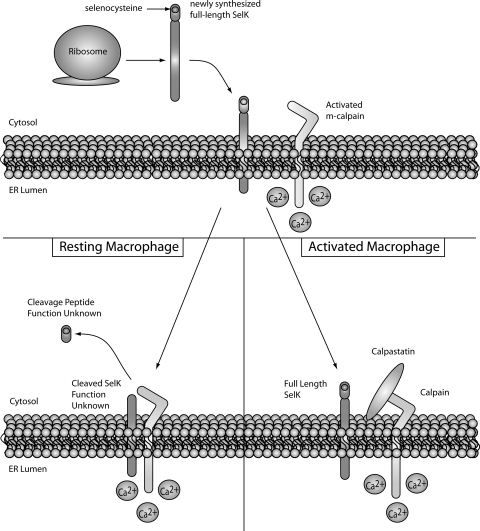
Selk cleavage by m-calpain in macrophages. In resting macrophages, Selk synthesized on the ribosome is immediately cleaved by activated m-calpain. This results in nearly all Selk existing as inactived protein in resting macrophages as demonstrated by lower Ca2+ flux and migration in response to chemokines such as MCP-1. TLR-activation increases expression of calpastatin, which inhibits cleavage by m-calpain and results in higher levels of full-length Selk. Thus, in activated macrophages, full-length Selk is able to efficiently promote Ca2+ and migration toward chemokines. MCP-1, monocyte chemotactic protein-1; TLR, Toll-like receptor.