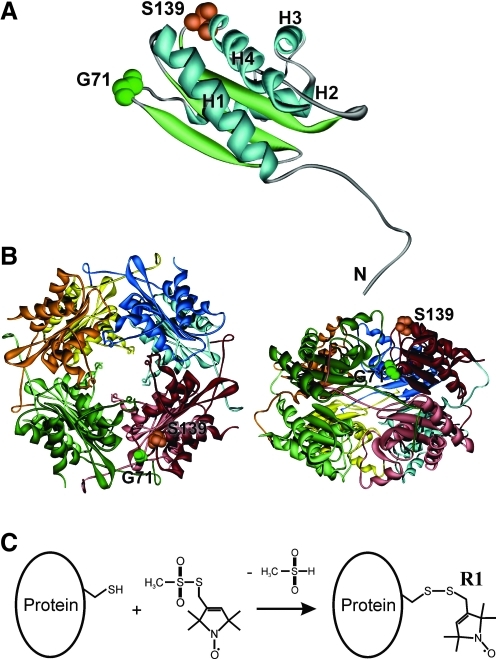
FIG. 1.
Positions of selected amino acids and site-directed spin labeling. (A) An image showing the amino acid residues Ser-139 (S139) and Gly-71 (G71) on the crystal structure of a single heme-binding protein (HbpS) unit (PDB: 3FPV). α-Helices are numbered H1–H4. The N-terminus is indicated. (B) Octomeric HbpS assembly viewed down the fourfold axis (left) and from the “side” (right) (PDB: 3FPV). A single HbpS monomer is highlighted. (C) Site-directed spin labeling. Reaction of the [(1-Oxyl-2,2,5,5-tetramethylpyrroline-3-methyl)methanethiosulfonate] spin label (MTSSL) with the sulfhydryl group of a cysteine side chain, generating the spin label side chain R1. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertonline.com/ars).