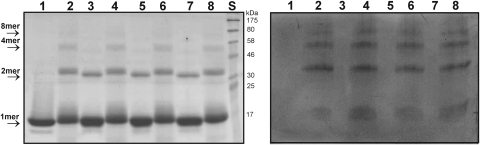
FIG. 2.
Iron-mediated crosslinking and carbonylation in HbpS proteins. Left: An aliquot of unstressed (wild-type [WT], lane 1; Ser139R, lane 3; Gly71R, lane 5; Ser139R-Gly71R, lane 7) or iron-stressed (WT, lane 2; Ser139R, lane 4; Gly71R, lane 6; Ser139R-Gly71R, lane 8) HbpS samples were subjected to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by Coomassie staining. Arrows indicate positions for the monomeric (1mer), dimeric (2mer), tetrameric (4mer), or octomeric (8mer) HbpS forms. The approximate sizes of prestained protein markers are also indicated (lane S). Right: A second aliquot of unstressed and iron-stressed samples were subjected to DNP derivatization as described by (26). Reaction products were loaded onto a 12% SDS-PAA gel (in the same order as in the gel showed in the left part of this figure). After electrophoresis, proteins were transferred on to a fluorotrans membrane and treated with anti-DNP antibodies as described earlier (26).