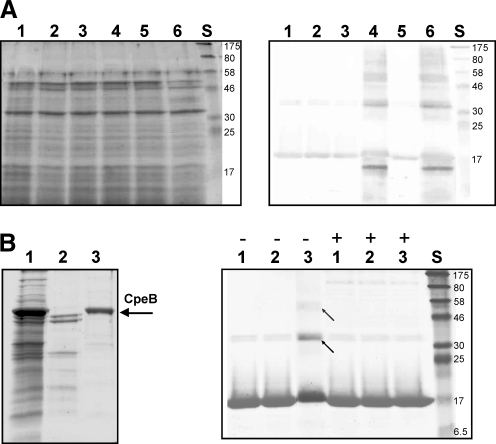
FIG. 7.
CpeB and the turnover of HbpS. (A) S. reticuli WT (lanes 1, 3 and 5) and S. reticuli ΔcpeB (lanes 2, 4 and 6) strains were cultivated in the presence of 1 mM FeCl2 previously treated with 10 mM DTT (see Material and Methods section). After 1 h (lanes 1 and 2), 2 h (lanes 3 and 4), and 3 h (lanes 5 and 6) of cultivation, the precipitated extracellular protein extracts were subjected to 12% SDS-PAGE, and either stained with Coomassie (left) or transferred to a fluorotrans membrane for Western analysis by using anti-HbpS antibodies to specifically identify HbpS within the protein extracts (right). The approximate sizes (in kDa) of prestained protein markers are also shown (lane S). (B, left) Mycelia-associated proteins were released from a Streptomyces strain over-producing CpeB (lane 1) by using 0.01% Triton X-100 (see Material and Methods section). After the third anion-exchange chromatography over a DEAE-Sepharose column (lane 2), CpeB was eluted from the DEAE-Sepharose column by using 100 mM NaCl (lane 3). The arrow indicates the position of CpeB on the 10% SDS PAA gel. (B, right) HbpS native (lane 1) or treated with FeCl2 and DTT in the presence (lane 2) or absence (lane 3) of EDTA were subjected to 12% SDS-PAGE and subsequently stained with Coomassie. The absence (−) or presence (+) of the isolated CpeB (see the left part of this figure) in the reactions is indicated. Crosslinked HbpS forms (lane 3,−) are indicated with arrows. The approximate sizes (in kDa) of prestained protein markers are also shown (lane S).