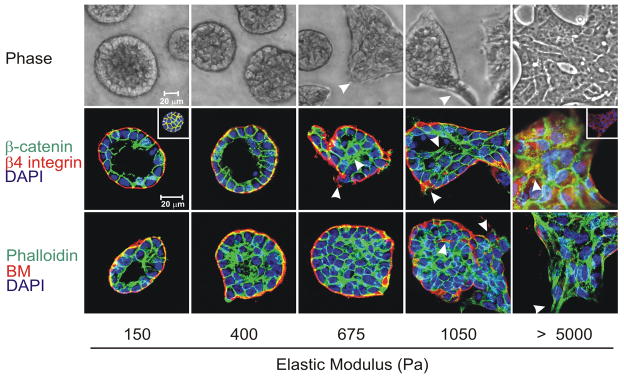
Figure 3.
Phase contrast microscopy and confocal immunofluorescence images of non-malignant immortalized human mammary epithelial cell (HMEC; MCF10A) colonies interacting with a three-dimensional reconstituted basement membrane (BM)-laminated polyacrylamide gel of increasing stiffness (150–5,000 Pa) showing colony morphogenesis after 20 days of culture. On compliant gels with materials properties similar to that measured in the normal murine mammary gland (150 Pa) non-malignant MECs proliferate for 6–12 days to eventually form growth-arrested, polarized acini analogous to the terminal ductal lobular units observed at the end buds of the differentiated breast. These structures have intact adherens junctions and insoluble cell–cell localized β-catenin before (main images) and after (inset a) Triton extraction, and polarity, as shown by the basal localization of (α6) β4 integrin, the apical–lateral localization of cortical actin (Phalloidin), and the assembly of an endogenous laminin 5 basement membrane. Incremental stiffening of the basement membrane gel progressively compromises tissue morphogenesis and alters EGF-dependent growth of these cells. Thus, colony size progressively increases with matrix stiffening, lumen formation is compromised, cell–cell junctions are disrupted, as revealed by loss of cell–cell-associated β-catenin (inset b), and tissue polarity is inhibited, as indicated by disorganized (α6) β4 integrin localization and loss of the endogenous laminin 5 basement membrane. Interestingly, actin stress fibers were not observed in the structures until the stiffness of the matrix reached 5,000 Pa, as has been observed in murine breast tumors in vivo. The arrows indicate loss of the endogenous basement membrane and disruption of basal polarity. Reproduced from [12, 14].