Summary
Epistaxis is a common disorder affecting equally both genders. Posterior origin of epistaxis in some instances requires endovascular treatment. Anastomoses between external carotid artery and internal carotid or ophthalmic arteries heighten the risk of stroke or blindness, if particles of polyvinyl alcohol are used for embolization. We report a case of 90-year-old man for whom successful embolization with N-Butyl Cyanoacrylate glue was performed as an alternative treatment for recurrent epistaxis.
Key words: epistaxis, endovascular, embolization, anastomoses, glue
Introduction
Epistaxis is one of the most common conditions in otolaryngology. It occurs in equal proportions in both sexes, and the incidence has a tendency to increase with age 1. Most cases manifest as anterior nasal bleeding, which often resolves spontaneously or can be treated conservatively. Posterior epistaxis, by contrast, may require emergent therapeutic measures such as posterior nasal packing or endovascular embolization. Polyvinyl alcohol (PVA) particles are most commonly used as embolic material 1,2. Care should be taken of anastomoses between external carotid artery (ECA) and internal carotid artery (ICA) or ophthalmic artery (OphA), which can, if embolized, cause serious complications such as stroke or blindness 1,3. We describe a case of recurrent spontaneous epistaxis in a 90-year-old man in whom n-butyl cyanoacrylate (NBCA) glue was used as an alternative embolic material to control nasal bleeding and avoid potential complications. The endovascular approach to this condition is described and the current literature reviewed.
Clinical History and Procedure
A 90-year-old man presented with incessant epistaxis, that did not resolve with simple measures, and thus required thorough otolaryngologycal examination. There was no history of facial trauma, coagulopathy or neoplasm. Laboratory tests revealed moderate anemia with decreased level of erythrocytes: 3.29×1012/L (normal level: 4.5-6.5×1012/L) and hemoglobin: 100 g/L (normal level: 130-180 g/L). Platelets were normal. Computed tomography of the head did not reveal any abnormality linked to epistaxis. The patient was initially treated with posterior nasal packing, and then had to be repacked as the bleeding did not stop. After failure of the second packing to control epistaxis, endovascular embolization was indicated as the next therapeutic option. Selective catheterization of the right and left ECA and ICA was performed through the right femoral approach using a 5 French catheter. No signs of carotid-cavernous fistula or aneurysm were observed on either ICA. An intense nasal arterial blush was demonstrated originating from the left sphenopalatine artery (SPA), and to a lesser extent from the right SPA (Figure 1). A Rebar 18 microcatheter was used for selective catheterization of the right and left SPA in a coaxial manner and under roadmap control. No anastomoses were seen between the SPA, facial artery (FA) and anterior and posterior ethmoid arteries (AEA, PEA) on either side during the superselective contrast injection. PVA particles of 250 to 355 microns were used for selective embolization of the right SPA. The patient started bleeding again through the nose during the procedure. Subsequently, embolization of the left SPA with 355 to 500 microns PVA particles was performed until left-sided arterial blush disappeared (Figure 1) and the bleeding stopped. The procedure was terminated at this time. In the recovery room epistaxis started again from the left nostril, and posterior packing was placed with a Foley catheter. The case was re-discussed, and re-embolization was considered. An angiogram and catheterization of the left ECA was performed using the same technique. An angiogram demonstrated patency of the left SPA and a mild nasal blush that was less conspicuous than on the previous post-embolization angiogram. However, opacification of the left ophA via small branches of the left SPA was identified with the presence of washout flow at the level of the left ICA (Figure 2). Given the risk of PVA particles embolization of the ophA and ICA, sealing of the left SPA with NBCA glue was then suggested. A 40% mixture of NBCA and Lipiodol was prepared. After safe positioning of the microcatheter in the most distal portion of the left SPA, a single injection of glue was carried out, which was enough to occlude the distal SPA. The left-sided nasal blush disappeared (Figure 3). No significant contribution from the left facial artery (FA) to the left-sided blush was noted. The posterior nasal packing was then removed, and no signs of active bleeding were seen during exploration of the nasopharynx. The patient was observed for the next several days in hospital without any further bleeding.
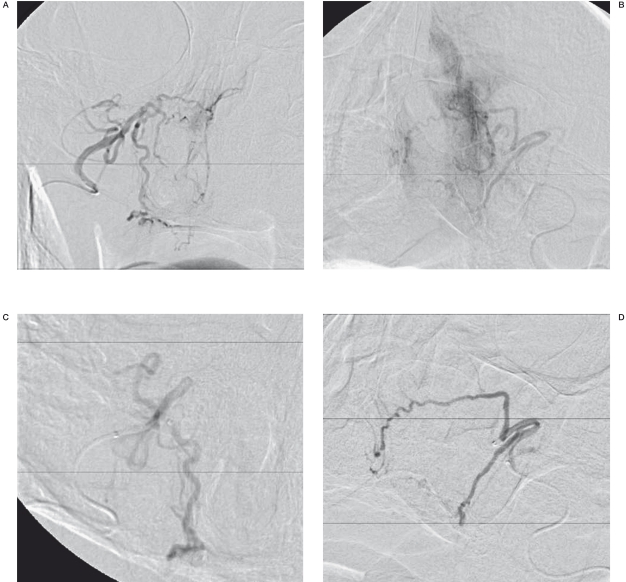
Figure 1.
Digital subtraction angiography (DSA). Frontal view. A,B) Arterial nasal blush is seen on both right and left sides, originating from sphenopalatine arteries. C,D) There is resolution of the nasal blush after selective embolization of the sphenopalatine artery on each side.
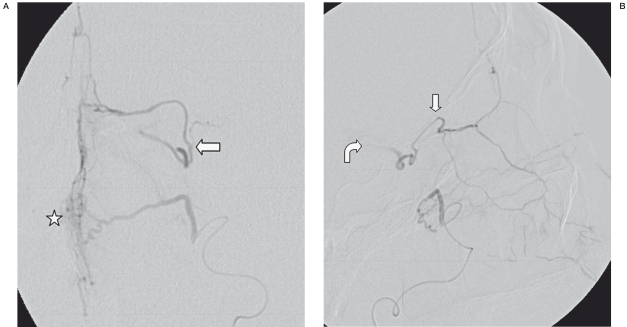
Figure 2.
SA. Frontal (A) and lateral (B) views of selective injection into the left sphenopalatine artery, one day after previous embolization, exhibit some nasal blush (asterisk), as well as collateral flow into the left ophthalmic artery (straight arrows). There is also mild washout of contrast into the left ICA on the lateral view (curved arrow).
Figure 3.
DSA. Frontal view of the left ECA after glue embolization demonstrates absence of collateral flow into the left ophthalmic artery and lack of nasal blush.
Discussion
Nasal bleeding is experienced by approximately 60% of adult people during their lifetime, but only 6% of them undergo medical treatment 4,5. Causes of epistaxis are various and include facial trauma, arteriovenous malformation, dural arteriovenous fistula, nontraumatic ICA aneurysm, hereditary hemorrhagic teleangietasia, coagulopathy, and neoplasms 1,2,5. However, most cases are of spontaneous nature, often related to inflammatory condition, arterial hypertension, atherosclerotic disease, and cigarette smoking 2. In about 80% of cases, epistaxis originates from the area of anterior nasal septa (Little area), and usually resolves spontaneously or may be easily treated with anterior nasal packing, cautery or diathermy 2,6. Failure of this initial therapy suggests posterior origin of bleeding which is less accessible for simple management, and often requires posterior nasal packing, endoscopic cauterization, or endovascular embolization 1,2. Transantral ligation of the internal maxillary artery (IMA) is an alternative therapeutic option with similar success rates, although, associated with higher overall rate of complications compared to the endovascular method 4,6,7.
The SPA, the terminal branch of IMA, provides the major vascular supply to the nasal fossa, and represents the main source of posterior nasal bleeding, and thus a usual target for embolization 1,2. The IMA is also commonly embolized 5. The floor of the nasal cavity recieves a combined supply from the ascending and descending palatine arteries, distal branches of the FA and IMA. The most anterior area is supplied by the superior labial artery, a distal branch of the FA. Finally the roof of the nasal cavity is supplied by the anterior and posterior ethmoid arteries (AEA, PEA), terminal branches of the OphA 1,2.
Frequently used embolic materials include gelatin sponge, gelfoam powder, PVA particles, platinum coils or their combination. Amongst them, PVA particles are most commonly used 1,2. The major complications of endovascular embolization arise from the presence of potentially dangerous anastomoses between the ECA and ICA or OphA, which may allow PVA particles to pass from the extracranial circulation into the intracranial circulation or ophA territory, compromising the arterial supply to the brain and eye, resulting in stroke or blindness 1-5. These anastomoses, when seen on an angiogram, may preclude endovascular treatment, especially with embolic materials that can reach far into the microcirculation. Collaterals with high potential for anastomosis include the middle meningeal artery, the accessory meningeal artery, the artery of the foramen rotundum, the inferolateral trunk, the meningohypophyseal trunk, the ascending pharyngeal artery, and communications between the SPA, FA and OphA 1.
Pre-embolization angiography of the ICA and ECA is mandatory to detect possible anatomical variants or vascular anomalies, and selectively guide the catheter to the region of interest under roadmap control. It is particularly important to look for communications between the SPA and ophA through AEA and PEA, as they represent a rete of terminal branches of the SPA and ophA. Choroidal blush from the ICA should be also verified before embolization, to exclude extracranial supply of the OphA 1,7. Appropriate positioning of the microcatheter, sufficiently distal to the origin of "dangerous" branches is essential to avoid non-target embolization 1,2,8.
For initial endovascular treatment of epistaxis, PVA particles certainly represent a first line embolic material, as they can appropriately and safely seal distal vascular branches of the mucosal stain. PVA particles of 150-500 μm are typically used. Smaller particles are expected to pass more easily through the anastomoses 7-9. Forceful injection of embolic material should be avoided, as it may lead to reflux with unintended embolization via more proximal anastomoses 1. Unfortunately, even appropriate position of the microcatheter may not prevent non-target embolization. In case of recurrent nasal bleeding PVL particles can also be used for embolization of the residual mucosal stain.
However, in the event that rebleeding is accompanied with coexistent findings or potentially threatening conditions, such as dangerous anastomoses, other safe embolic material should be considered. Coils or NBCA may be used in these cases for the permanent occlusion of the main arterial trunk (i.e. SPA).
Platinum coils can adequately seal vessels to control epistaxis. Pushable coils are probably less suitable because of potential coil embolism and, thus, were not under consideration in the presented case. Detachable coils, however, can be safely employed.
NBCA glue may represent an alternative option to other embolic materials. NBCA has been widely used for embolization of intracranial arteriovenous malformations, indirect carotid cavernous fistulas, and, occasionally, cerebral aneurysms 10-11. Only a few cases of the use of NBCA glue in endovascular management of epistaxis have been reported in the literature 3,12.
The benefit of NBCA was also confirmed in several traumatic instances 11. Due to its liquid nature, NBCA may potentially extend to small distal branches under certain circumstances, such as injection under flow arrest with further flow re-establishing following withdrawal of a microcatheter. However, NBCA in a sufficient high concentration provides an adequate sealing of larger proximal vessels, with minimal risk of penetration into small branches and distal embolization, especially in a low flow situation, also diminishing the risk of mucosal necrosis.
The embolization method using NBCA glue also allows to reduce the duration of the procedure due to the easier and faster embolization technique, and avoid multiple catheter manipulation if only one vessel needs to be embolized 3. In the presented case we preferred NBCA to other embolic agent, as we felt comfortable using NBCA at a high enough concentration given the flow velocity on the preembolic angiographic road-mapping test. We were able to occlude the distal SPA and isolate it from the circulation by a single injection of NBCA, without evidence of recurrent bleeding. We presumed that the revascularization of the residual mucosal stain from the ophA would not be enough to cause a significant rebleeding, based on the fact that the SPA remained the main provider for posterior nasal circulations in our patient, while the contribution of the ophA was minimal. The potential disadvantage of this technique is that NBCA does not reach the microcirculation, and may therefore be less effective when faced with a possible rich collateral circulation of the target.
In conclusion, NBCA glue may be considered a good alternative to PVA in selected cases of recurrent epistaxis following initial embolization, when anatomical variations preclude the use of particles.
References
- 1.Willems PW, Farb RI, Agid R. Endovascular treatment of epistaxis. Am J Neuroradiol. 2009;30:1637–1645. doi: 10.3174/ajnr.A1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koh E, Frazzini VI, Kagetsu NJ. Epistaxis: vascular anatomy, origins, and endovascular treatment. Am J Roentgenol. 2000;174:845–851. doi: 10.2214/ajr.174.3.1740845. [DOI] [PubMed] [Google Scholar]
- 3.Mahadevia AA, Murphy KJ, Obray R, et al. Embolization for intractable epistaxis. Tech Vasc Interv Radiol. 2005;8:134–138. doi: 10.1053/j.tvir.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Tseng EY, Narducci CA, Willing SJ, et al. Angiographic embolization for epistaxis: a review of 114 cases. Laryngoscope. 1998 Apr;108(4 Pt 1):615–619. doi: 10.1097/00005537-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Christensen NP, Smith DS, Barnwell SL, et al. Arterial embolization in the management of posterior epistaxis. Otolaryngol Head Neck Surg. 2005 Nov;133(5):748–753. doi: 10.1016/j.otohns.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Vokes DE, McIvor NP, Wattie WJ, et al. Endovascular treatment of epistaxis. ANZ J Surg. 2004;74:751–753. doi: 10.1111/j.1445-1433.2004.03145.x. [DOI] [PubMed] [Google Scholar]
- 7.Ernst RJ, Bulas RV, Gaskill-Shipley M, et al. Endovascular therapy of intractable epistaxis complicated by carotid artery occlusive disease. Am J Neuroradiol. 1995;16:1463–1468. [PMC free article] [PubMed] [Google Scholar]
- 8.Elden L, Montanera W, Terbrugge K, et al. Angiographic embolization for the treatment of epistaxis: a review of 108 cases. Otolaryngol Head Neck Surg. 1994 Jul;111(1):44–50. doi: 10.1177/019459989411100110. [DOI] [PubMed] [Google Scholar]
- 9.Ashwin PT, Mirza S, Ajithkumar N, et al. Iatrogenic central retinal artery occlusion during treatment for epistaxis. Br J Ophthalmol. 2007;91:122–123. doi: 10.1136/bjo.2006.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng MM, Chen CC, Lirng JF, et al. N-butyl-2-cyanoacrylate for embolization of carotid aneurysm. Neuroradiology. 1994;36:144–147. doi: 10.1007/BF00588084. [DOI] [PubMed] [Google Scholar]
- 11.Luo CB, Teng MM, Chang FC, et al. Transarterial balloon-assisted n-butyl-2-cyanoacrylate embolization of direct carotid cavernous fistulas. Am J Neuroradiol. 2006 Aug;27(7):1535–1540. [PMC free article] [PubMed] [Google Scholar]
- 12.Merland JJ, Melki JP, Chiras J, et al. Place of embolization in the treatment of severe epistaxis. Laryngoscope. 1980;90:694–704. [PubMed] [Google Scholar]