Figure 6.
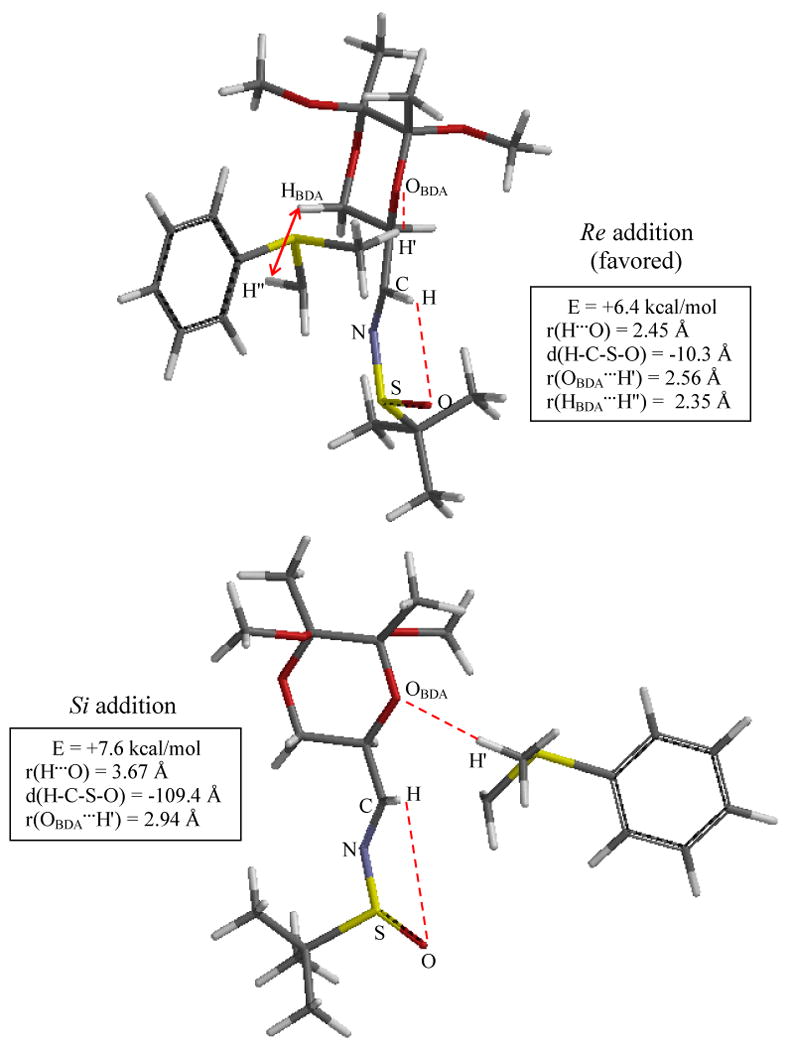
Optimized TS structures for Additions onto Imine 5 using Sulfur Ylide 1. Structures and energies determined at the B3LYP/6-311+G**(THF) level of theory; energies are ZPE-corrected and relative to reactants. Significant interaction distances are indicated. Top: The R configuration of the sulfinyl sulfur permits ylide approach to the Re face; Re face approach is favored in spite of a steric clash with BDA. Bottom: The sulfinyl oxygen/iminyl hydrogen interaction is disrupted to permit Si approach, but Si approach occurs without steric clash with BDA. Imine 5's N- and S- substituents are not matched, with the former favoring Re addition and dominant, while the latter favors Si addition.
