Abstract
Background
To assess the long-term cost-effectiveness of rosuvastatin therapy compared with generic simvastatin and generic atorvastatin in reducing the incidence of cardiovascular events and mortality in a Swedish population with Framingham risk ≥20%.
Methods
A probabilistic Monte Carlo simulation model based on data from JUPITER (the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) was used to estimate the long-term cost-effectiveness of rosuvastatin 20 mg daily versus simvastatin or atorvastatin 40 mg for the prevention of cardiovascular death and morbidity. The three- stage model included cardiovascular event prevention simulating the 4 years of JUPITER, initial prevention beyond the trial, and subsequent cardiovascular event prevention. A Swedish health care payer perspective (direct costs only) was modeled for a lifetime horizon, with 2008/2009 as the costing period. Univariate and probabilistic sensitivity analyses were performed.
Results
The incremental cost per quality-adjusted life-year (QALY) gained with rosuvastatin 20 mg over simvastatin or atorvastatin 40 mg ranged from SEK88,113 (rosuvastatin 20 mg versus simvastatin 40 mg; Framingham risk ≥30%; net avoidance of 34 events/1000 patients) to SEK497,542 (versus atorvastatin 40 mg: Framingham risk ≥20%; net avoidance of 11 events/1000 patients) over a lifetime horizon. Probabilistic sensitivity analyses indicated that at a willingness-to-pay threshold of SEK500,000/QALY, rosuvastatin 20 mg would be cost-effective for approximately 75%–85% of simulations relative to atorvastatin or simvastatin 40 mg. Sensitivity analyses indicated the findings to be robust.
Conclusion
Rosuvastatin 20 mg is cost-effective over a lifetime horizon compared with generic simvastatin or atorvastatin 40 mg in patients at high cardiovascular risk in Sweden.
Keywords: cardiovascular disease, cost-benefit analysis, cost-effectiveness, rosuvastatin, simvastatin, atorvastatin, generic, high risk
Introduction
The objective of cardiovascular disease management is now well established in the prevention of myocardial infarction, stroke, and cardiovascular death. Current treatment guidelines, including the US National Cholesterol Education Program Adult Treatment Panel III and updates1 and the European consensus guidelines,2,3 recommend statin therapy for patients with established vascular disease, diabetes, and hyperlipidemia. A 10% reduction in plasma levels of total cholesterol is followed by a 25% reduction in the incidence of coronary artery disease after 5 years, and a reduction in low-density lipoprotein cholesterol of 1 mmol/L is accompanied by a 20% reduction in coronary events.3–5 Current European guidelines suggest statin therapy for patients with a persistent SCORE (Systematic COronary Risk Evaluation) risk ≥5% despite lifestyle changes and dietary and exercise therapy.3 SCORE is a more recently developed risk assessment system aimed at European clinical practice and is based on 12 European cohort studies, allowing estimation of 10-year cardiovascular risk in ostensibly healthy persons.6 Swedish guidelines are also based on SCORE risk and recommend the use of a statin for at-risk patients who do not attain blood lipid goals (total cholesterol < 5.0 mmol/L and low-density lipoprotein cholesterol < 3.0 mmol/L, with more stringent targets for patients in certain very high-risk groups) with lifestyle, dietary, and exercise therapy.7
Despite these recommendations, approximately half of all myocardial infarctions and strokes are seen in persons with low-density lipoprotein cholesterol levels below the recommended thresholds for drug treatment.8 A recent clinical trial, ie, JUPITER (Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) demonstrated that rosuvastatin 20 mg daily significantly reduced the incidence of major cardiovascular events (CVEs) and all-cause mortality among individuals without hyperlipidemia but with elevated high-sensitivity C-reactive protein levels.9 These effects were similar in all subgroups, including patients at intermediate and high coronary disease risk (Framingham risk scores ≥10%).
The objective of the present study was to estimate the long-term cost-effectiveness of rosuvastatin 20 mg compared with atorvastatin 40 mg or simvastatin 40 mg in reducing major CVEs and mortality using a model previously developed on the basis of the JUPITER results.10 The JUPITER population included patients with a baseline high-sensitivity C-reactive protein level > 2 mg/L. For the current model, we used the relative risk reduction observed in JUPITER to predict CVEs for different populations with varying levels of baseline cardiovascular risk (estimated based on Framingham risk score11), irrespective of baseline high-sensitivity C-reactive protein levels. The Framingham risk score is recommended in clinical guidelines as a tool to predict the absolute risk of coronary events in populations free of cardiovascular disease. It is calculated using gender, age, smoking status, total cholesterol, high-density lipoprotein, systolic blood pressure, and diabetes status. The Framingham risk score indicates the risk level for a coronary heart disease event occurring within the following 10 years. The current analysis has a long-term (lifetime) time horizon and is based on patients with high baseline cardiovascular risk (≥20% or ≥30% Framingham), which is in line with new European labeling recommendations. As indicated by current guidelines for cost-effectiveness analyses in Sweden, a long-term time horizon was utilized to capture the full costs and clinical effects of treatment for chronic conditions.12 Specifically, study time frames are required to cover the period when the main health effects and costs arise. Extrapolation beyond the time frame of clinical trials is therefore required, which is carried out via modeling. For the analyses presented here, clinically relevant dosages of rosuvastatin and two other statins were compared over lifetime and 20-year time horizons.
Materials and methods
The cost-effectiveness model used is based on the outcomes of JUPITER, and can analyze CVE reduction with rosuvastatin 20 mg daily relative to no treatment,10 or can be used for active comparisons between differing dosages of rosuvastatin and other statins. Under the latter scenario, CVE reduction with the non-rosuvastatin comparators are approximated on the basis of their effect relative to rosuvastatin 20 mg in reducing the ratio of total serum cholesterol to HDL cholesterol because the utility of this measure is established in the literature.13,14
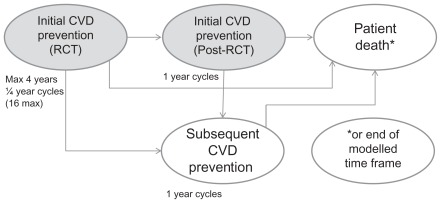
The model incorporates the primary endpoints of JUPITER, ie, fatal and nonfatal myocardial infarction, fatal and nonfatal stroke, coronary arterial revascularization (coronary artery bypass grafting [CABG] or percutaneous transluminal coronary angioplasty/stent [PTCA]), unstable angina, or death from cardiovascular causes. Noncardiovascular death and fatal and nonfatal venous thromboembolic events (both deep vein thrombosis and pulmonary embolism) are also included. The 4-year time frame of JUPITER is simulated, with continuation beyond this to a stage simulating initial prevention beyond the trial time frame (initial CVE prevention-post RCT [randomized controlled trial]), and finally to a subsequent event prevention stage, applied after a patient has an initial CVE (secondary CVE prevention, see Figure 1). A probabilistic first-order Monte Carlo micro-simulation model estimates the long-term cost-effectiveness of treatment.
Figure 1.
Model structure overview.
Abbreviations: CVD, cardiovascular disease; RCT, randomized controlled trial.
In this study, the simulation model assessed cost-effectiveness from a Swedish health systems payer perspective through the incorporation of direct medical costs for the years 2008/2009. The base case comparison was between rosuvastatin 20 mg and generic simvastatin 40 mg or generic atorvastatin 40 mg over a patient’s lifetime for persons in Sweden with a Framingham risk ≥20% or ≥30%.
Model structure
Simulations are performed with a cycle length of 3 months during the RCT stage and subsequently on a one-year cycle length until the patient’s death or attainment of age 100 years, or until the end of the specified model time frame. All patients start by entering the CVE prevention-RCT stage for up to 4 years. They then have an event probability (derived from the JUPITER data) on a 3-month basis from which they transfer to the subsequent prevention stage (initial CVE prevention-post RCT stage). The events are: nonfatal myocardial infarction, nonfatal stroke, unstable angina, CABG, or PTCA, which are followed by transition to the next stage of the model (secondary CVE prevention stage); cardiovascular death, noncardiovascular death or venous thromboembolic death, all of which occasion transition to the patient death stage; and 16 quarters (4 years) without a clinical endpoint, which is followed by retransition to the initial CVE prevention-post RCT stage. Nonfatal venous thromboembolism is possible in the CVE prevention-RCT stage, with accrual of associated costs, but does not force a transition because it is not considered to be a CVE. Patients without events stay in the initial prevention stage until the next cycle of the model. For patients who discontinue treatment, the effect of treatment on event transition probabilities is phased out over 5 years (20% per year) as per the long-term analysis of the West of Scotland Coronary Prevention Study.15 Treatment-persistent patients are assumed to have a 5% annual discontinuation probability, based on studies of statin persistence under usual care.16 Further details of the model have been published previously.10
Age-based CVE rates for the secondary CVE prevention stage were based on those reported in the National Institute for Health and Clinical Excellence Health Technology Assessment 2007 report.17 Venous thromboembolism rates and relative risks were carried forward from the CVE prevention- RCT to the post-RCT stages. Noncardiovascular death rates were calculated in the same manner in the RCT and post-RCT stages of the model. Only one nonfatal CVD event per patient was allowed in each one-year cycle, although a single nonfatal venous thromboembolism was allowed with a nonfatal CVE.
Clinical events: primary and secondary CVE prevention
Events captured by the model were as already described. Quarter by quarter probabilities of experiencing an initial event with rosuvastatin 20 mg were calculated by dividing the JUPITER trial-adjudicated quarterly event counts by the number of patients at risk at the beginning of a quarter for the first 4 years of the model (CVE prevention-RCT). These findings were used to construct survival curves for the trial period. Data were fitted to exponential curves with R2 values exceeding 0.97, from which were calculated constant time-based event probabilities. The relative risk of a primary CVE with rosuvastatin 20 mg treatment was then calculated from the ratio of treated and untreated event probabilities, and was determined to be 0.49 for patients at higher risk. This was used instead of the JUPITER trial relative risk of 0.56, as the constant quarterly event probability calculation accounted for the shape of the curve (slope and height), providing an improved estimate for carrying forward over the long term, and the high R2 value of the fitted exponential curves provided the justification for utilizing constant treatment relative risk values of a CVE during both the modeled trial and post-RCT stages.
JUPITER data were also used to calculate probability distributions of major CVEs. Arterial revascularization was treated as a single event, assuming an 80:20 split between PTCA and CABG. The rate of venous thromboembolism was also derived from JUPITER.18 Venous thromboembolic events were modeled as a weighted combination of deep vein thrombosis and pulmonary embolism events. Noncardiovascular death rates were calculated using Swedish life tables.19 The annual probabilities of an initial event were adjusted and carried forward from the CVE prevention-RCT stage of the model to the initial CVE prevention-post RCT stage. The baseline probability was subsequently increased annually on the basis of Framingham 10-year risk age-adjustment calculations,20 which yielded a model default age-based risk increase of approximately 5% per year. The relative risks of an event with rosuvastatin treatment, distribution of events, and venous thromboembolic event rates were all carried forward from the CVE prevention-RCT stage. Annual noncardiovascular death rates were calculated in the same manner as for the RCT stage.
Treatment continuation
During the CVE prevention-RCT stage of the model, the probability of a patient remaining on treatment declined linearly over the 4-year period to 75%, which corresponded to the discontinuation rate observed in the JUPITER trial. Accordingly, discontinuation did not affect effectiveness during this stage, because the impact of discontinuation is already reflected in the efficacy estimates from the clinical trial data. However, discontinuation did affect the estimated treatment costs by reflecting reduced drug use. For the secondary CVE prevention stage of the model, treatment costs reflected decreased drug utilization, and the treatment relative risk was phased back to unity (relative risk = 1.0) over a five-year period for both the CVE prevention-post- RCT and subsequent CVE prevention stages.
Clinical events: nonrosuvastatin 20 mg comparators
CVE reduction effects were approximated on the basis of their effect on total cholesterol:HDL cholesterol ratios relative to rosuvastatin 20 mg. The algorithm used to calculate relative risks was based on the Framingham risk equation of Anderson et al.21 Default total cholesterol:HDL cholesterol reduction inputs were taken from Jones et al22 and from Schneck et al.23 Cardiovascular risk over 10 years was calculated for an untreated population using the Framingham risk equation, and was then repeated for the identical populations being treated with the various statins on the basis of total cholesterol:HDL cholesterol reductions. The event rates versus rosuvastatin 20 mg were calculated as ratios of the resulting 10-year cardiovascular risk. Population characteristics defining modeled populations that are used as inputs in the Framingham risk equation are age, total cholesterol, HDL cholesterol, systolic blood pressure, diabetes, and smoking. Default values corresponded to the overall JUPITER population.9
Costs and cost-effectiveness
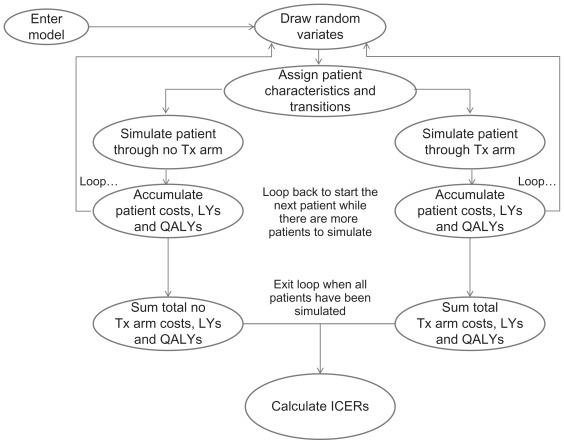
Costs, event counts, life-years gained, and quality-adjusted life-years (QALYs) were accumulated at each stage of the model for each cohort of patients (Figure 2). Costs included those associated with treatment (drugs, physician visits, and monitoring tests) and those related to events (hospitalization and physician visits associated with coronary events).
Figure 2.
Model cohort simulation.
Abbreviations: ICER, incremental cost effectiveness ratio; LYs, life-years; QALYs, quality-adjusted life-years; Tx, treatment.
Prescription drug costs were obtained from Swedish public health sources, including the Swedish Association of Local Authorities and Regions,24 the Karolinska Institute,25 and Swedish medical literature.26 Predicted future rosuvastatin costs based on future availability of generic products were estimated by assuming a 95% reduction in price within one year of a generic becoming available. The specific event cost estimates used in the model are summarized in Table 1.
Table 1.
Direct medical cost estimates for cardiovascular events
| Cardiovascular event direct costs (2008/2009 Swedish kronor) | Event year | Subsequent years | Source |
|---|---|---|---|
| NonFatal MI | 164,296.16 | 43,781.84 | (25) |
| PTCA | 69,700.00 | 0 | (24) |
| CABG | 167,000.00 | 0 | (24) |
| Unstable angina | 149,510.89 | 44,642.81 | (25) |
| Nonfatal stroke | 163,205.00 | 57,304.00 | (26)a |
| Cardiovascular death (secondary prevention) | 17,762.00 | 0 | (24) |
| Noncardiovascular death | Mexico ratios of cardiovascular death to noncardiovascular death cost | 0 | |
| By cardiovascular death type (primary prevention) | |||
| MI death | 17,762.00 | 0 | (24) |
| Stroke death | 17,762.00 | 0 | (24) |
| Other cardiovascular death | 17,762.00 | 0 | (24) |
Note: Costs inflated to 2009 values.
Abbreviations: MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty; CABG, coronary artery bypass graft.
Incremental cost-effectiveness ratios (ICERs) were calculated using the formula where effect is measured in either life-years or QALYs:
Utilities
All simulated patients had an assigned initial baseline age-adjusted utility value based on values reported in the literature,27 which was updated as the patient aged through the model. Utility weights for each model event were based on estimates reported in the literature28–30 (Table 2). Multiplicative utility calculations were performed where patients had experienced more than one event and multiple disutility values were to be applied (the assumed “joint” utility value was the product of the individual utility values). Discounting with half-cycle correction of life-years, QALYs, and costs was performed with 3% annual discount rates.
Table 2.
Disutilities for CVD events
| Event year | Subsequent years | |
|---|---|---|
| Nonfatal MI disutility28 | 0.24 | 0.24 |
| PTCA disutility29 | 0.0175 | 0 |
| CABG disutility29 | 0.037 | 0 |
| Unstable angina disutility28 | 0.23 | 0 |
| Nonfatal stroke disutility30 | 0.37 | 0.37 |
| CVD death disutility | 0.5 | 1.0 |
| Non-CVD death disutility | 0.5 | 1.0 |
Abbreviations: CABG, coronary artery bypass graft; CVD, cardiovascular disease; MI, Myocardial infarction; PTCA, Percutaneous transluminal coronary angioplasty.
Sensitivity analyses
One-way sensitivity analyses were carried out by decreasing (50%) or increasing (50% and 100%) secondary statin treatment costs, varying rosuvastatin discontinuation rates (to 50% over 4 years), varying proportions of patients starting secondary prevention therapy and subsequently discontinuing, decreasing (50%) or increasing (50% or 100%) event costs, altering (±50%) event disutilities, varying the discount rate between 0% and 5%, varying the primary CVE risk (±50%), and varying the primary prevention relative risk of CVE with rosuvastatin (±50%). The effect of reducing the time horizon of the model to 20 years was also examined, as were the effects of no future introduction of generic rosuvastatin and the adoption of the JUPITER relative risk of 0.56 (rather than 0.49) for a primary event (≥20% Framingham risk only). The effect of increasing the dosage of rosuvastatin to 40 mg daily and atorvastatin to 80 mg daily in patients at highest risk (≥30%) was also investigated. Probabilistic sensitivity analyses were performed for event costs, event disutilities, and relative risk of events. For costs, log-normal distributions were used because costs tend to be skewed with a long tail at the high end. Full details are published elsewhere.10
Results
The ICERs for rosuvastatin are summarized in Table 3. For patients with a 10-year Framingham risk ≥20%, lifetime costs per QALY gained were SEK497,542 versus atorvastatin 40 mg and SEK151,323 versus simvastatin 40 mg. Corresponding costs per QALY in patients with ≥30% risk were SEK342,403 (versus atorvastatin) and SEK88,113 (versus simvastatin). Relative to atorvastatin 40 mg, with ≥20% and ≥30% risk, respectively, approximately 11 and 14 CVEs were avoided over the projected lifetimes of 1000 patients, with 5.21 and 6.9 cardiovascular deaths, 5.29 and 7.0 nonfatal myocardial infarctions, 2.89 and 3.16 nonfatal strokes, and 1.87 and 2.32 PTCAs being avoided.
Table 3.
Base case analysis for cost-effectiveness of rosuvastatin vs generic atorvastatin and simvastatin: lifetime horizon in patients with Framingham risk ≥20% or ≥30%. Results per 1000 patients with 95% generic price reduction assumption
| Parameter | Difference between RSV and comparator dose | |||
|---|---|---|---|---|
| ≥20% Risk | ≥30% Risk | |||
| RSV20 vs SMV40 | RSV20 vs ATV40 | RSV20 vs SMV40 | RSV20 vs ATV40 | |
| Total life-years gained (LYG) | 97.32 | 41.61 | 132.00 | 58.18 |
| Total quality-adjusted life-years (QALYs) gained | 95.15 | 40.90 | 129.50 | 56.33 |
| Total direct costs (SEK) | 14,398,515 | 20,348,033 | 11,411,059 | 19,286,798 |
| Cost per LYG (ICER) [SEK] | 147,952 | 489,047 | 86,448 | 331,503 |
| Cost per QALY gained (ICER) [SEK] | 151,323 | 497,542 | 88,113 | 342,403 |
Abbreviations: ATV, atorvastatin; ICER, incremental cost-effectiveness ratio; LYG, life-year gained; QALY, quality adjusted life-year; SEK, Swedish kronor; RSV, rosuvastatin; SMV, simvastatin.
Relative to simvastatin 40 mg with ≥20% risk, approximately 26 CVEs were avoided with the use of rosuvastatin over the projected lifetimes of 1000 patients, with 11.95 cardiovascular deaths, 13.48 nonfatal myocardial infarctions, 5.86 nonfatal strokes, and 4.72 PTCAs being avoided. Corresponding figures for ≥30% risk were 34 CVEs, with 15.71 cardiovascular deaths, 18.17 nonfatal myocardial infarctions, 7.02 nonfatal strokes, and 5.63 PTCAs avoided. For every 1000 modeled at-risk patients, the incremental direct medical cost of rosuvastatin over comparator statins at the dosages used in the model varied from approximately SEK11.5 million to SEK20 million, with an estimated 40.90 to 129.50 QALYs gained over a lifetime (Table 3).
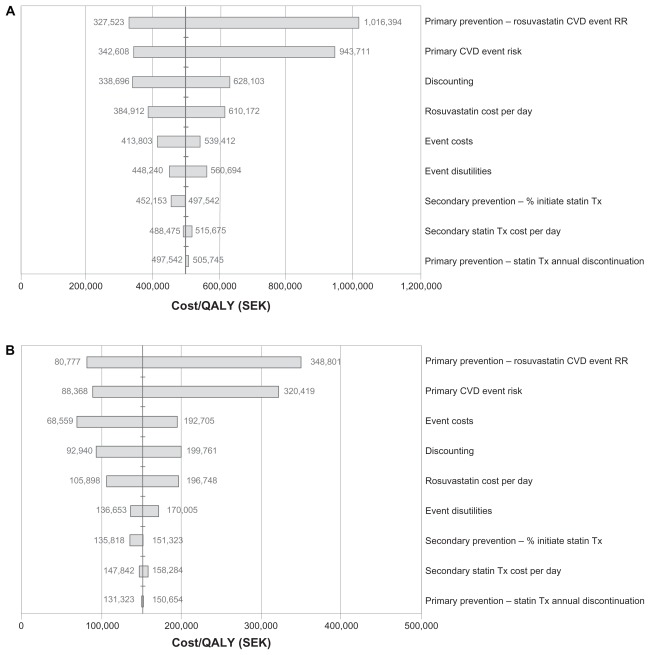
Sensitivity analyses were applied to assess the change in the ICER estimates across a wide range of statin drug costs, event costs, risks, discontinuation rates, discounting, and utility values. The results of the one-way sensitivity analysis for the lifetime horizon in patients with a Framingham risk ≥20% are summarized in the tornado diagrams in Figure 3. In all scenarios, the variable with the greatest effect on estimated cost-effectiveness was the assumed treatment effectiveness of rosuvastatin in the initial prevention stage. In ≥20% risk patients in the atorvastatin comparison, an assumed 50% increase in rosuvastatin CVE relative risk (0.49 to 0.735, ie, decreased effectiveness) approximately doubled the ICER to SEK1,016,394, whereas a 50% decrease (0.49 to 0.245, ie, increased effectiveness) reduced the ICER to SEK327,523. A similar effect was seen in the simvastatin comparison, where a 50% increase in rosuvastatin CVE relative risk increased the ICER to SEK348,801, and a 50% decrease reduced the ICER to SEK80,777. For patients at ≥30% risk, a 50% increase in rosuvastatin CVE relative risk increased the ICERs to SEK702,023 versus atorvastatin and to SEK231,574 versus simvastatin, whereas a 50% decrease in rosuvastatin CVE relative risk reduced the respective ICERs to SEK254,950 and SEK54,996 (data not shown). The primary CVE risk was the second most important influence on estimated cost effectiveness with both comparisons and in both risk groups. Some variation between groups was seen for the relative importance of the remaining factors, with the applied discount rate, cost of CVEs, and daily cost of rosuvastatin being most influential across groups.
Figure 3.
(A) One-way sensitivity analysis of lifetime horizon for Framingham 20% risk population (JUPITER population): rosuvastatin 20 mg versus atorvastatin 40 mg assuming a 95% generic price reduction from brand. (B) One-way sensitivity analysis of lifetime horizon for Framingham 20% risk population (JUPITER population): rosuvastatin 20 mg versus simvastatin 40 mg assuming a 95% generic price reduction from brand.
Abbreviations: CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; SEK, Swedish kronor; Tx, treatment; JUPITER, the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; RR, relative risk; QALY, quality adjusted life-year.
As shown in Table 4, shortening of the time horizon to 20 years decreased the incremental cost-effectiveness of rosuvastatin relative to simvastatin and atorvastatin. At ≥20% risk, the effect of no future introduction of generic rosuvastatin was similar to that of restricting the time horizon to 20 years, whereas an increase in relative risk of a primary event (use of JUPITER relative risk of 0.56) was associated with slight reductions in ICERs relative to the base case comparisons (Table 4). Modeling of increased dosages in patients at highest risk (≥30%) increased the ICER over the base case. The ICER of rosuvastatin 40 mg relative to atorvastatin 80 mg over a lifetime horizon was SEK493,003, ie, somewhat higher than the base case ICER of SEK342,403 for rosuvastatin 20 mg versus atorvastatin 40 mg in these high-risk patients.
Table 4.
Cost-effectiveness of rosuvastatin (RSV) vs atorvastatin (ATV) and simvastatin (SMV): effect of alteration of time horizon, no future introduction of generic rosuvastatin, and increase in cardiac event relative risk
| Parameter | Drug comparison | |||
|---|---|---|---|---|
| ≥20% Risk | ≥30% Risk | |||
| RSV20 vs SMV40 | RSV20 vs ATV40 | RSV20 vs SMV40 | RSV20 vs ATV40 | |
| 20-year time horizon | 198,415 | 640,708 | 112,545 | 423,036 |
| No introduction of generic rosuvastatin | 216,924 | 650,169 | 133,359 | 446,428 |
| Cardiac event relative risk 0.56 | 132,257 | 445,792 | 73,141 | 298,318 |
Abbreviations: ATV, atorvastatin; ICER, incremental cost-effectiveness ratio; RSV, rosuvastatin; SEK, Swedish kronor; SMV, simvastatin.
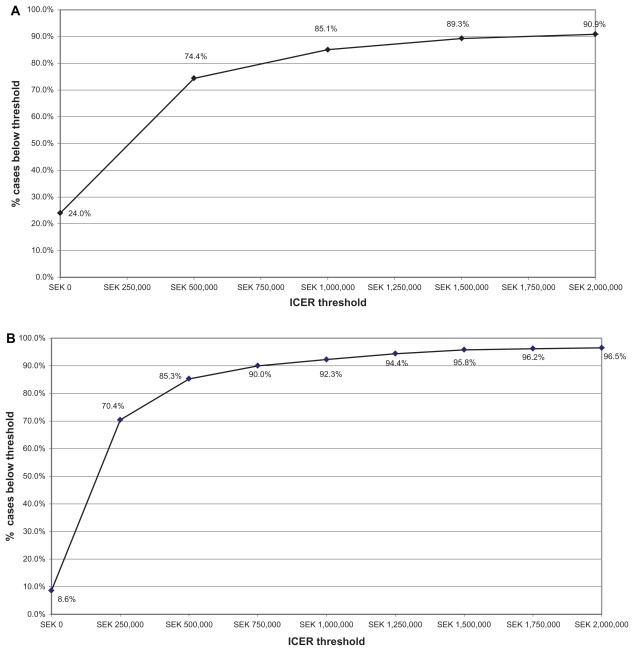
Probabilistic sensitivity analysis was performed to assess the decision uncertainties in multiple model parameters. The cost-effectiveness acceptability curves generated (Figure 4) for different levels of willingness to pay for rosuvastatin 20 mg relative to atorvastatin or simvastatin 40 mg in patients with a Framingham risk ≥20% indicate that, at a maximum willingness to pay threshold of SEK500,000 per QALY gained, rosuvastatin therapy is cost-effective in 74.4% and 85.3% of the model replications, respectively. At a Framingham risk ≥30%, rosuvastatin is cost-effective for 86.9% and 90.3% of model replications when compared with atorvastatin and simvastatin, respectively, at this willingness to pay threshold. At ≥20% risk, rosuvastatin is estimated to be cost-saving versus atorvastatin and simvastatin, respectively, in 24.0% and 8.6% of model replications, and in 29.0% and 14.8% of replications at the ≥30% level.
Figure 4.
(A) Willingness to pay-cost/QALY ICER with a lifetime horizon for Framingham 20% risk population (JUPITER population) rosuvastatin 20 mg versus atorvastatin 40 mg, assuming a generic 95% price reduction from brand. (B) Willingness to pay-cost/QALY ICER with a lifetime horizon for Framingham 20% risk population (JUPITER population) rosuvastatin 20 mg versus simvastatin 40 mg, generic 95% price reduction from brand.
Abbreviations: QALY, quality adjusted life year; ICER, incremental cost-effectiveness ratio; SEK, Swedish kronor; JUPITER, the Justification for the Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin.
Discussion
The comparisons carried out in the present study indicate that rosuvastatin 20 mg is likely to be cost-effective over generic atorvastatin or simvastatin 40 mg in terms of reducing cardiovascular mortality and morbidity among patients at moderate to high cardiovascular risk over a patient’s lifetime. The simulations modeled indicated overall ICERs (cost/QALY) associated with the use of rosuvastatin versus the other statins ranging from SEK88,113 (versus simvastatin 40 mg at ≥30% 10-year risk) to SEK497,542 (versus atorvastatin 40 mg at ≥20% 10-year risk). The time horizon for this analysis extends beyond the anticipated market entry of generic rosuvastatin, and the current analysis therefore accounts for this as in previous work based on the JUPITER trial.10 Accounting for future generic drug costs is recognized as increasing the reliability of estimates of the true cost-effectiveness of a medical intervention.31–33
The cost-effectiveness of rosuvastatin was driven by cardiovascular events avoided. Deaths avoided tended to be counterbalanced to some extent by increases in noncardiovascular deaths with rosuvastatin relative to atorvastatin and simvastatin at the dosages examined, but this was likely to be intrinsically attributable to this increased avoidance of cardiovascular death. Patients who avoid premature death due to cardiovascular causes have an increased likelihood of dying from other noncardiovascular causes. This effect will increase in magnitude as the time horizon expands, and will be maximally expressed over a lifetime horizon.
Sensitivity analyses indicate that the incremental costs per QALY gained (or ICERs for short) were fairly robust with respect to time horizons of 20 years or longer. The probabilistic sensitivity analysis for the lifetime horizon for a dosage of rosuvastatin 20 mg versus simvastatin or atorvastatin 40 mg indicated that, at a maximum willingness to pay a threshold of SEK500,000, rosuvastatin treatment would be acceptable in approximately 75%–85% of simulations.
The World Health Organization has suggested international cost-effectiveness threshold values of three times the gross domestic product per capita,34 and thresholds up to US$100,000 have been suggested.35,36 In Sweden, values equivalent to around US$100,000 (about €70,000) have been indicated on the basis of willingness to pay for prevention of road deaths.37 Based on the average exchange rate for USD to SEK in 2008/09 (7.123) this indicates equivalence to approximately SEK712,300 which would encompass all the quality-adjusted lifetime horizon estimates generated by the comparisons carried out here.
We note also that the model as applied in the present study focused on at-risk patient populations with Framingham scores ≥20% or ≥30%. The model used the relative risk of 0.49 for the at-risk population rather than the JUPITER relative risk of 0.56. This value was used because it gave the best representation of the curve for cardiac event risk and was the best estimate to carry forward over the long-term model. However, previously published data have shown this relative risk to yield similar results to the relative risk of 0.56 when used for the at-risk population.10 The use of patient populations with high Framingham risk scores reflects the need for more aggressive statin treatments in higher-risk populations to address their higher unmet medical need,1 because elevated cardiovascular risk represents an economic burden to health care providers. To explore further the use of more aggressive therapy, we also modeled rosuvastatin 40 mg versus atorvastatin 80 mg over a lifetime in patients at highest risk who might require higher daily dosages to achieve their lipid goals. Although there was an increase in the ICER of rosuvastatin under this scenario, which was driven by an increase in cost associated with drug therapy, the ICER generated was still below SEK500,000 and within the limits of what would be considered cost-effective in Sweden. The effect of no future introduction of generic rosuvastatin and restriction of the time horizon to 20 years also yielded ICERs for rosuvastatin that remained within the boundaries of what is likely to be considered cost-effective.
The results of the present study build on the findings of the previous cost-effectiveness comparison of rosuvastatin and placebo in patients at moderate to high risk,10 and suggest that this agent is likely to be cost-effective relative to atorvastatin or simvastatin, as well as relative to no statin treatment. Our scenario accounts for the likely introduction within the projected time horizons of generic rosuvastatin, which increases confidence in the cost estimates. The methodological strength of the comparisons is improved by the use of the ratio between total and HDL cholesterol levels in serum as a calibration factor, given that total cholesterol:HDL cholesterol is reported to be a reliable predictor of cardiovascular risk.13,14
As with all cost-effectiveness models, ours is subject to limitations that merit consideration. Various input parameters were estimated and derived from the literature or other publicly available data sources, which by their nature, introduce inherent uncertainty. As is standard practice, we attempted to assess robustness of results against parameter estimate uncertainties by performing sensitivity analyses. We used Swedish life tables to estimate cardiovascular and noncardiovascular mortality, which is relevant, given differences in treatment patterns in different countries (eg, criteria for coronary interventional procedures).38–41 JUPITER used a placebo comparison group, and the first published use of the present model simulated initial prevention based upon a placebo comparison. Both analyses to date have been carried out from a direct health care payer’s perspective, and indirect costs covering lost wages or productivity have not been included. Indirect costs would be of interest and would reflect increased incidences of coronary events leading, for example, to lost work time in patients treated with placebo because of increased incidences of coronary events. They would also show up societal effects of differences in efficacy and adverse event profiles between statins. Thus, future work would benefit from inclusion of indirect (societal) costs, especially given the lifetime perspective of the simulations.
Rosuvastatin 20 mg daily is therefore likely to be a cost-effective intervention for the long-term management of coronary risk when compared with either placebo (ie, no treatment)10 or with atorvastatin or simvastatin 40 mg daily in patients with elevated risk of coronary disease. The current inter-statin estimates as presented here are limited to direct medical costs in Sweden, and future research should expand estimates to include other countries and payer perspectives, most notably societal (indirect) costs over the lifetime horizon, which would only add to the benefits of rosuvastatin (and therefore make rosuvastatin an even more cost-effective alternative.
Footnotes
Disclosure
The research was sponsored by AstraZeneca. SG and TP are employees of AstraZeneca. MJ was an employee of AstraZeneca at the time of the research. LS and KF have served as consultants to AstraZeneca. AO has received grants for clinical research from and has served as a consultant to AstraZeneca. Editorial support in the preparation of the manuscript was provided by Mapi Values, UK, and funded by AstraZeneca.
References
- 1.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 2.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24:1601–1610. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 3.Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 4.Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31:236–244. doi: 10.1016/j.clinthera.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 7.Läkemedelsverket (Medical Products Agency) Förebyggande av aterosklerotisk hjärt-kärlsjukdom. Version. 2006. [Accessed May 9, 2011]. p. 3. Available from: http://www.lakemedelsverket.se/upload/halso-och-sjukvard/behandlingsrekommendationer/080313_primarprevention.pdf.
- 8.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Ohsfeldt RL, Gandhi SK, Smolen LJ, et al. Cost effectiveness of rosuvastatin in patients at risk of cardiovascular disease based on findings from the JUPITER trial. J Med Econ. 2010;13:428–437. doi: 10.3111/13696998.2010.499758. [DOI] [PubMed] [Google Scholar]
- 11.National Institutes of Health. National Institutes of Health Publication No. 02-5215. Sep, 2002. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final Report. [Google Scholar]
- 12.Tandvårds-och Läkemedelsformånsverket (Swedish Dental and Pharmaceutical Benefits Agency) General Guidelines for Economic Evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2) [Accessed November 9, 2011]. Available from: http://www.tlv.se.
- 13.Holman RR, Coleman RL, Shine BS, Stevens RJ. Non-HDL cholesterol is less informative than the total-to-HDL cholesterol ratio in predicting cardiovascular risk in type 2 diabetes. Diabetes Care. 2005;28:1796–1797. doi: 10.2337/diacare.28.7.1796. [DOI] [PubMed] [Google Scholar]
- 14.Tohidi M, Hatami M, Hadaegh F, Safarkhani M, Harati H, Azizi F. Lipid measures for prediction of incident cardiovascular disease in diabetic and non-diabetic adults: results of the 8.6 years follow-up of a population based cohort study. Lipids Health Dis. 2010;9:6. doi: 10.1186/1476-511X-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford I, Murray H, Packard CJ, Shepherd J, MacFarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357:1477–1486. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 16.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 17.Ward S, Lloyd JM, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11:1–160. iii–iv. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 18.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–1861. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statistika Centralbrån (Statistics Sweden) [Accessed May 9, 2011]. Available from: http://www.scb.se/
- 20.The Framingham Heart Study. Framingham Heart Study, Coronary Heart Disease (10-year risk) [Accessed May 9, 2011]. Available from: http://www.framinghamheartstudy.org/risk/coronary.html.
- 21.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121(1 Pt 2):293–298. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 22.Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26:1388–1399. doi: 10.1016/j.clinthera.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Schneck DW, Knopp RH, Ballantyne CM, McPherson R, Chitra RR, Simonson SG. Comparative effects of rosuvastatin and atorvastatin across their dose ranges in patients with hypercholesterolemia and without active arterial disease. Am J Cardiol. 2003;91:33–41. doi: 10.1016/s0002-9149(02)02994-6. [DOI] [PubMed] [Google Scholar]
- 24.Swedish Association of Local Authorities and Regions. [Accessed May 9, 2011]. Available from: http://www.skl.se/web/Hem.aspx.
- 25.Sigvant B. Department of Molecular Medicine and Surgery. Stockholm, Sweden: Karolinska Institute; 2009. [Accessed May 9, 2011]. Epidemiological aspects of peripheral arterial disease. Available from: http://diss.kib.ki.se/2009/978-91-7409-670-5/thesis.pdf. [Google Scholar]
- 26.Ghatnekar O, Persson U, Glader EL, Terent A. Cost of stroke in Sweden: an incidence estimate. Int J Technol Assess Health Care. 2004;20:375–380. doi: 10.1017/s0266462304001217. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan PW, Arant TW, Ellis SL, Ulrich H. The cost effectiveness of anticoagulation management services for patients with atrial fibrillation and at high risk of stroke in the US. Pharmacoeconomics. 2006;24:1021–1033. doi: 10.2165/00019053-200624100-00009. [DOI] [PubMed] [Google Scholar]
- 28.Goodacre S, Nicholl J, Dixon S, et al. Randomized controlled trial and economic evaluation of a chest pain observation unit compared with routine care. BMJ. 2004;328:254. doi: 10.1136/bmj.37956.664236.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scuffham PA, Kosa J. The cost-effectiveness of fluvastatin in Hungary following successful percutaneous coronary intervention. Cardiovasc Drugs Ther. 2006;20:309–317. doi: 10.1007/s10557-006-8877-3. [DOI] [PubMed] [Google Scholar]
- 30.Tengs TO, Lin TH. A meta-analysis of quality-of-life estimates for stroke. Pharmacoeconomics. 2003;21:191–200. doi: 10.2165/00019053-200321030-00004. [DOI] [PubMed] [Google Scholar]
- 31.Shih YC, Han S, Cantor SB. Impact of generic drug entry on cost-effectiveness analysis. Med Decis Making. 2005;25:71–80. doi: 10.1177/0272989X04273139. [DOI] [PubMed] [Google Scholar]
- 32.Hoyle M. Future drug prices and cost-effectiveness analyses. Pharmacoeconomics. 2008;26:589–602. doi: 10.2165/00019053-200826070-00006. [DOI] [PubMed] [Google Scholar]
- 33.Pharmaceutical Management Agency. Prescription for pharmacoeconomic analysis: methods for cost-utility analysis. May, 2007. [Accessed May 9, 2011]. Available from: http://www.pharmac.govt.nz/2007/06/19/PFPAFinal.pdf.
- 34.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 35.Cutler DM, Rosen AB, Vijan S. The value of medical spending in the United States, 1960–2000. N Engl J Med. 2006;355:920–927. doi: 10.1056/NEJMsa054744. [DOI] [PubMed] [Google Scholar]
- 36.Blomstrom P, Ekman M, Lundqvist CB, et al. Cost effectiveness of cardiac resynchronization therapy in the Nordic region: an analysis based on the CARE-HF trial. Eur J Heart Fail. 2008;10:869–877. doi: 10.1016/j.ejheart.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Persson U, Hjelmgren J. Health services need knowledge of how the public values health. Lakartidningen. 2003;100:3436–3437. Swedish. [PubMed] [Google Scholar]
- 38.Carlhed R, Bojestig M, Peterson A, et al. Improved clinical outcome after acute myocardial infarction in hospitals participating in a Swedish quality improvement initiative. Circ Cardiovasc Qual Outcomes. 2009;2:458–464. doi: 10.1161/CIRCOUTCOMES.108.842146. [DOI] [PubMed] [Google Scholar]
- 39.National Health Statistics Reports. No. 5, July 30, 2008. 2006 National Hospital Discharge Survey. [Accessed May 9, 2011]. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr005.pdf.
- 40.National Adult Cardiac Surgical Database Report 1999–2000. The United Kingdom Cardiac Surgical Register. [Accessed May 9, 2011]. Available from: http://www.scts.org/file/NACSDreport2000ukcsr.pdf.
- 41.British Cardiovascular Intervention Society. United Kingdom national PCI audit data. [Accessed May 9, 2011]. Available from: http://www.bcis.org.uk/pages/page_box_contents.asp?pageid=697&navcatid=11.