Abstract
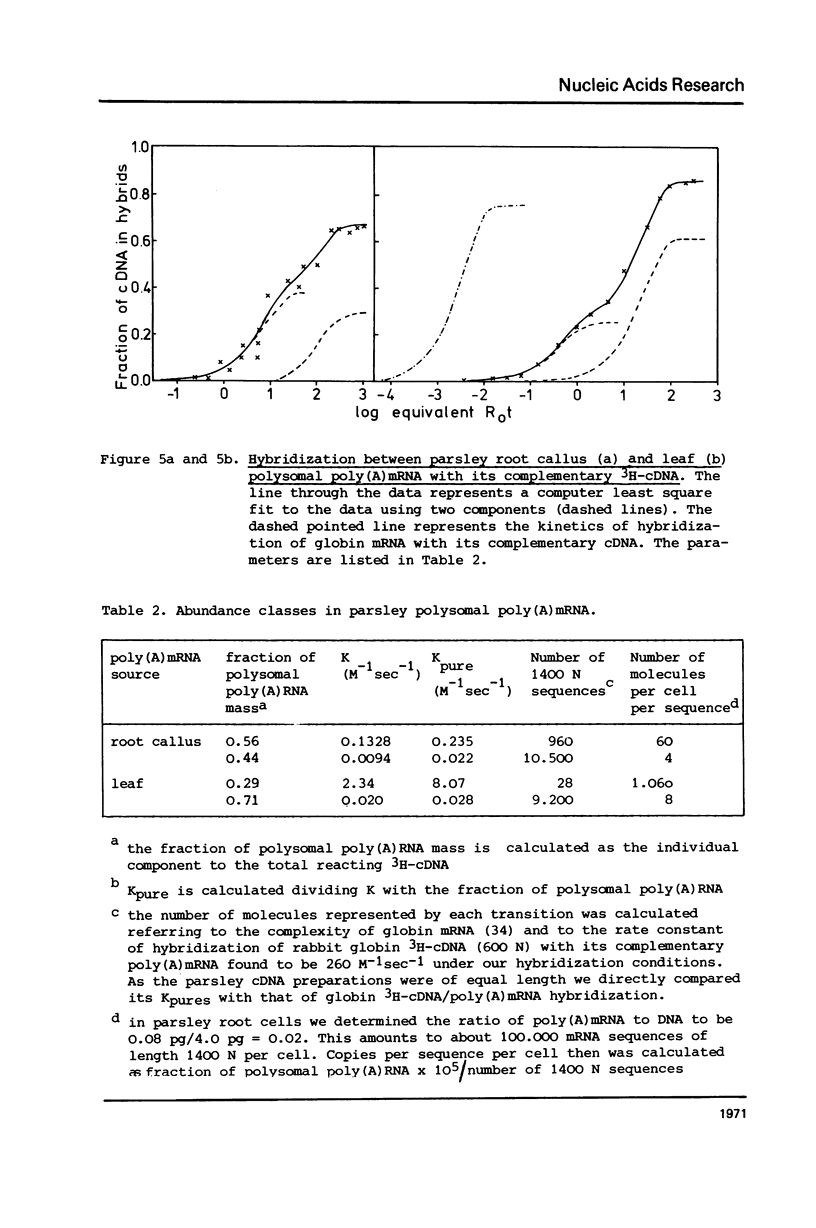
Representation of genomic kinetic sequence classes and sequence complexities were investigated in nuclear and polysomal RNA of the higher plant Petroselinum sativum (parsley). Two different methods indicated that most if not all polysomal poly(A) -RNA is transcribed from unique sequences. As measured by saturation hybridization in root callus and young leaves 8.7% and 6.2%, respectively, of unique DNA were transcribed in mRNA corresponding to 13.700 and 10.000 average sized genes. Unique nuclear DNA hybridized with an excess of polysomal poly(A)mRNA to the same extent as with total polysomal RNA. 3H-cDNA - poly(A)mRNA hybridization kinetics revealed the presence of two abundance classes with 9.200 and about 30 different mRNAs in leaves and two abundance classes with 10.500 and 960 different mRNAs in callus cells. The existence of plant poly(A)hnRNA was proven both by its fast kinetics of appearance, its length distribution larger than mRNA, and its sequence complexity a few times that of polysomal RNA.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Bantle J. A., Maxwell I. H., Hahn W. E. Specificity of oligo (dT)-cellulose chromatography in the isolation of polyadenylated RNA. Anal Biochem. 1976 May 7;72:413–427. doi: 10.1016/0003-2697(76)90549-2. [DOI] [PubMed] [Google Scholar]
- Birnie G. D., MacPhail E., Young B. D., Getz M. J., Paul J. The diversity of the messenger RNA population in growing Friend cells. Cell Differ. 1974 Nov;3(4):221–232. doi: 10.1016/0045-6039(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Bourque D. P., Naylor A. W. A simple electrophoretic procedure for separation of RNA on mixed agarose-acrylamide gel columns. J Chromatogr. 1971 Mar 24;56(1):79–86. doi: 10.1016/s0021-9673(00)97779-3. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Bishop J. O. Two classes of messenger RNA in cultured rat cells: repetitive sequence transcripts and unique sequence transcripts. J Mol Biol. 1974 Dec 25;90(4):649–663. doi: 10.1016/0022-2836(74)90530-0. [DOI] [PubMed] [Google Scholar]
- Chapman K. S., Ingle J. The stability, polyadenylic acid content and ribonucleoprotein form of nulcear ribonucleic acid in artichoke. Biochem J. 1976 Dec 1;159(3):585–600. doi: 10.1042/bj1590585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Sack G. H., Jr, Nathans D. Studies of simian virus 40 DNA. VII. A cleavage map of the SV40 genome. J Mol Biol. 1973 Aug 5;78(2):363–376. doi: 10.1016/0022-2836(73)90122-8. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Amenson C. S., Britten R. J. General interspersion of repetitive with non-repetitive sequence elements in the DNA of Xenopus. J Mol Biol. 1973 Jun 15;77(1):1–23. doi: 10.1016/0022-2836(73)90359-8. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Flavell R. B., Bennett M. D., Smith J. B., Smith D. B. Genome size and the proportion of repeated nucleotide sequence DNA in plants. Biochem Genet. 1974 Oct;12(4):257–269. doi: 10.1007/BF00485947. [DOI] [PubMed] [Google Scholar]
- Friedman E. Y., Rosbash M. The syntheiss of high yields of full-length reverse transcripts of globin mRNA. Nucleic Acids Res. 1977 Oct;4(10):3455–3471. doi: 10.1093/nar/4.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Klein W. H., Davis M. M., Wold B. J., Britten R. J., Davidson E. H. Structural gene sets active in embryos and adult tissues of the sea urchin. Cell. 1976 Apr;7(4):487–505. doi: 10.1016/0092-8674(76)90200-2. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Smith M. J., Britten R. J., Davidson E. H. Studies on nucleic acid reassociation kinetics: retarded rate of hybridization of RNA with excess DNA. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2306–2310. doi: 10.1073/pnas.74.6.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Birnie G. D., Young B. D., MacPhail E., Paul J. A kinetic estimation of base sequence complexity of nuclear poly(A)-containing RNA in mouse Friend cells. Cell. 1975 Feb;4(2):121–129. doi: 10.1016/0092-8674(75)90118-x. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Kamalay J. C., Timberlake W. E. Sequence complexity of nuclear and polysomal RNA in leaves of the tobacco plant. Cell. 1978 May;14(1):123–131. doi: 10.1016/0092-8674(78)90307-0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Bishop J. O. The expression of three abundance classes of messenger RNA in mouse tissues. Cell. 1976 Dec;9(4 Pt 2):761–774. doi: 10.1016/0092-8674(76)90139-2. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Brown I. R. Analysis of rabbit brain polysomal poly (A+) mRNA by DNA excess hybridization. Biochim Biophys Acta. 1977 Jan 3;474(1):141–153. doi: 10.1016/0005-2787(77)90221-0. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Rosbash M. Regulation of a set of abundant mRNA sequences. Cell. 1977 Mar;10(3):463–467. doi: 10.1016/0092-8674(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Hough B. R., Smith M. J., Britten R. J., Davidson E. H. Sequence complexity of heterogeneous nuclear RNA in sea urchin embryos. Cell. 1975 Jul;5(3):291–299. doi: 10.1016/0092-8674(75)90104-x. [DOI] [PubMed] [Google Scholar]
- Key J. L., Silflow C. The occurrence and distribution of poly(a) ribonucleic Acid in soybean. Plant Physiol. 1975 Sep;56(3):364–369. doi: 10.1104/pp.56.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiper M. A quick hydroxyapatite chromatography technique especially adapted for work with DNA networks. Anal Biochem. 1978 Nov;91(1):70–74. doi: 10.1016/0003-2697(78)90816-3. [DOI] [PubMed] [Google Scholar]
- Klein W. H., Murphy W., Attardi G., Britten R. J., Davidson E. H. Distribution of repetitive and nonrepetivite sequence transcripts in HeLa mRNA. Proc Natl Acad Sci U S A. 1974 May;71(5):1785–1789. doi: 10.1073/pnas.71.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: the relationship between heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Jan;4(1):11–20. doi: 10.1016/0092-8674(75)90128-2. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Manning J. E., Schmid C. W., Davidson N. Interspersion of repetitive and nonrepetitive DNA sequences in the Drosophila melanogaster genome. Cell. 1975 Feb;4(2):141–155. doi: 10.1016/0092-8674(75)90121-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melli M., Whitfield C., Rao K. V., Richardson M., Bishop J. O. DNA-RNA hybridization in vast DNA excess. Nat New Biol. 1971 May 5;231(18):8–12. [PubMed] [Google Scholar]
- Ohta T., Kimura M. Functional organization of genetic material as a product of molecular evolution. Nature. 1971 Sep 10;233(5315):118–119. doi: 10.1038/233118a0. [DOI] [PubMed] [Google Scholar]
- Ragg H., Schroeder J., Hahlbrock K. Poly(A)-containing RNA from Petroselinum hortense: isolation, properties and messenger function in vitro. Mol Biol Rep. 1975 Jul;2(2):119–127. doi: 10.1007/BF00357542. [DOI] [PubMed] [Google Scholar]
- Ragg H., Schröder J., Hahlbrock K. Translation of poly(A)-containing and poly(A)-free messenger RNA for phenylalanine ammonia-lyase, a plant-specific protein, in a reticulocyte lysate. Biochim Biophys Acta. 1977 Jan 20;474(2):226–233. doi: 10.1016/0005-2787(77)90197-6. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Ford P. J., Bishop J. O. Analysis of the C-value paradox by molecular hybridization. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3746–3750. doi: 10.1073/pnas.71.9.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage M. J., Sala-Trepat J. M., Bonner J. Measurement of the complexity and diversity of poly(adenylic acid) containing messenger RNA from rat liver. Biochemistry. 1978 Feb 7;17(3):462–467. doi: 10.1021/bi00596a014. [DOI] [PubMed] [Google Scholar]
- Tautvydas K. J. Mass isolation of pea nuclei. Plant Physiol. 1971 Apr;47(4):499–503. doi: 10.1104/pp.47.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timberlake W. E., Shumard D. S., Goldberg R. B. Relationship between nuclear and polysomal RNA populations of Achlya: a simple eucaryotic system. Cell. 1977 Apr;10(4):623–632. doi: 10.1016/0092-8674(77)90095-2. [DOI] [PubMed] [Google Scholar]
- Williamson R., Morrison M., Lanyon G., Eason R., Paul J. Properties of mouse globin messenger ribonucleic acid and its preparation in milligram quantities. Biochemistry. 1971 Aug 3;10(16):3014–3021. doi: 10.1021/bi00792a005. [DOI] [PubMed] [Google Scholar]
- Woo S. L., Dugaiczyk A., Tsai M. J., Lai E. C., Catterall J. F., O'Malley B. W. The ovalbumin gene: cloning of the natural gene. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3688–3692. doi: 10.1073/pnas.75.8.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]


