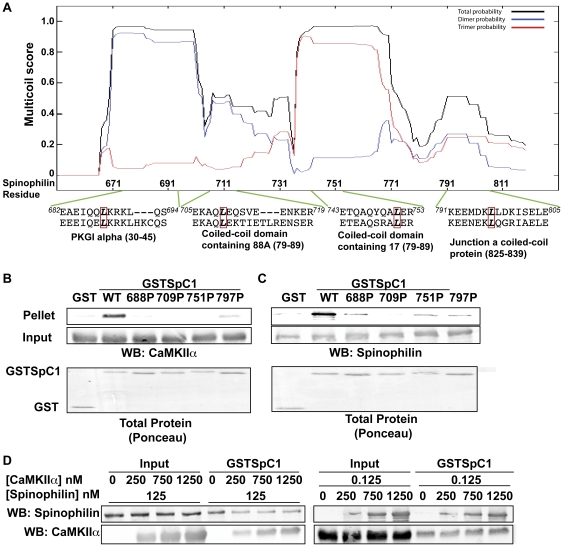
Figure 3. Role of C-terminal coiled-coil domains of spinophilin.
A. The MultiCoil algorithm was used to predict coiled-coil domain structures in the C-terminal domain of spinophilin. Amino acid sequences of the four predicted coiled-coil domains in spinophilin were aligned with amino acid sequences of coiled-coil domains in other proteins as indicated below. Residues 682–694 with mouse PKGI alpha; residues 705–719 with mouse coiled-coil domain containing 88A; residues 741–751 with mouse coiled-coil domain containing 17; residues 791–805 with mouse junction a coiled coil protein. A red box indicates conserved leucines that were mutated to proline. These mutations were predicted to selectively disrupt each of the coiled-coil domains using Multicoil (Fig. S1). B. Non- or Thr286-autophosphorylated CaMKIIα was incubated with WT or mutated (L688P, L709P, L751P, or L797P) GSTSpC1. Complexes were isolated using glutathione agarose and GST precipitates were immunoblotted for CaMKII. The total protein stain (Ponceau) of GST or GST proteins is also shown. C. His-tagged full-length, WT spinophilin was incubated with WT or mutated (L688P, L709P, L751P, or L797P) GSTSpC1. GST precipitates were isolated using glutathione agarose and immunoblotted for spinophilin. Total protein stain (Ponceau) of GST or GST proteins is also shown D. Non phosphorylated CaMKII or spinophilin was incubated GSTSpC1 along with increasing concentrations of either spinophilin or CaMKII. Complexes were isolated using glutathione agarose and immunoblotted for spinophilin and CaMKII. Figures are representative of at least 2 experiments.