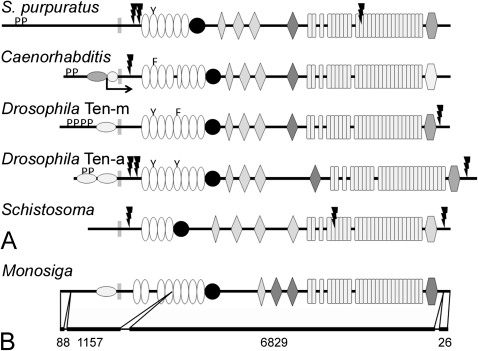
FIG. 5.
The domain organization of nonchordate teneurins. The ECDs of teneurins from the purple sea urchin (Strongylocentrotus purpuratus), Caenorhabditis elegans, Drosophila melanogaster, and the trematode Schistosoma mansoni have the same basic organization as chordate teneurins, but there is variation in the number of EGF repeats. Aromatic residues substitute for cysteines in at least one EGF repeats in all the species examined except the fluke, which suggests that the teneurin from S. mansoni does not dimerize (A). The genome of the choanoflagellate Monosiga brevicollis encodes a protein with a domain organization that is identical to metazoan teneurins. The predicted protein does not have furin cleavage sites or SH3-binding domains, and its EGF repeats contain a full complement of cysteines. The predicted M. brevicollis teneurin is encoded on just four exons, and most of the ECD is encoded on a single mega-exon of 6829 bp (B).