Abstract
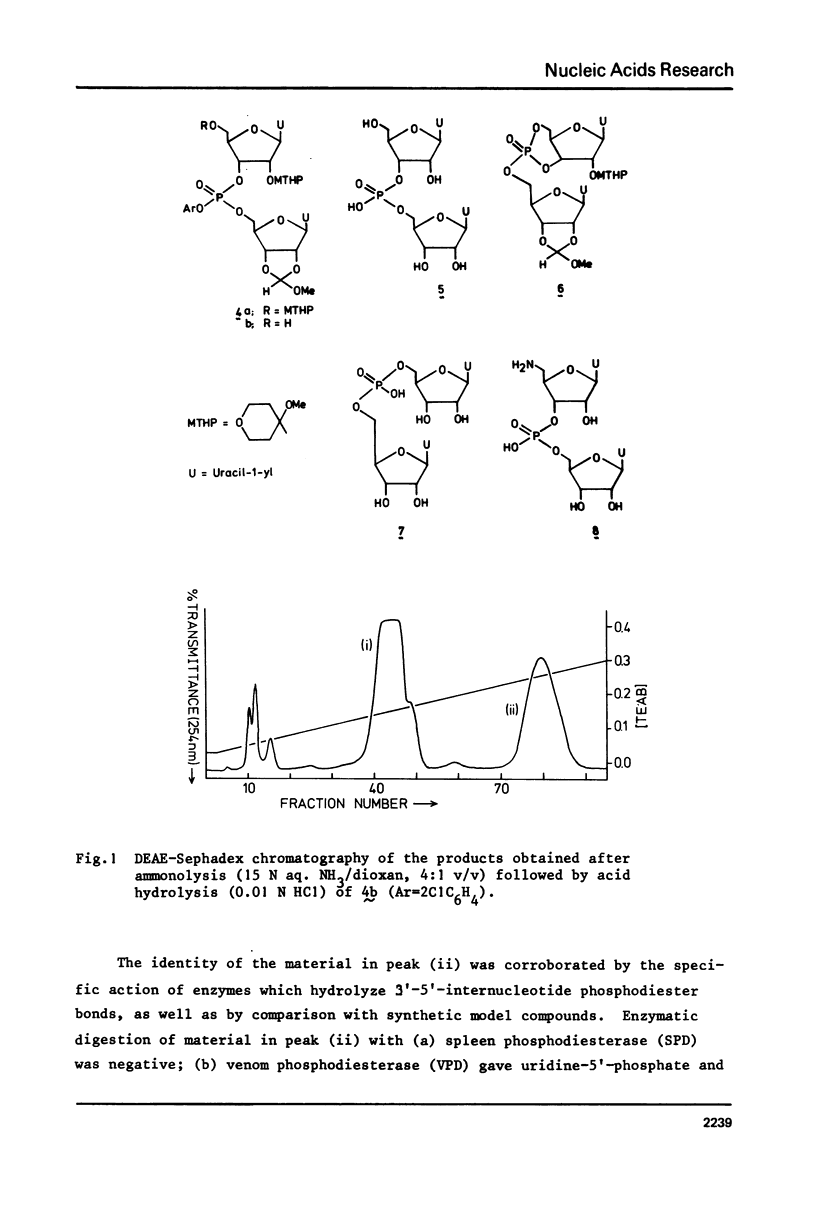
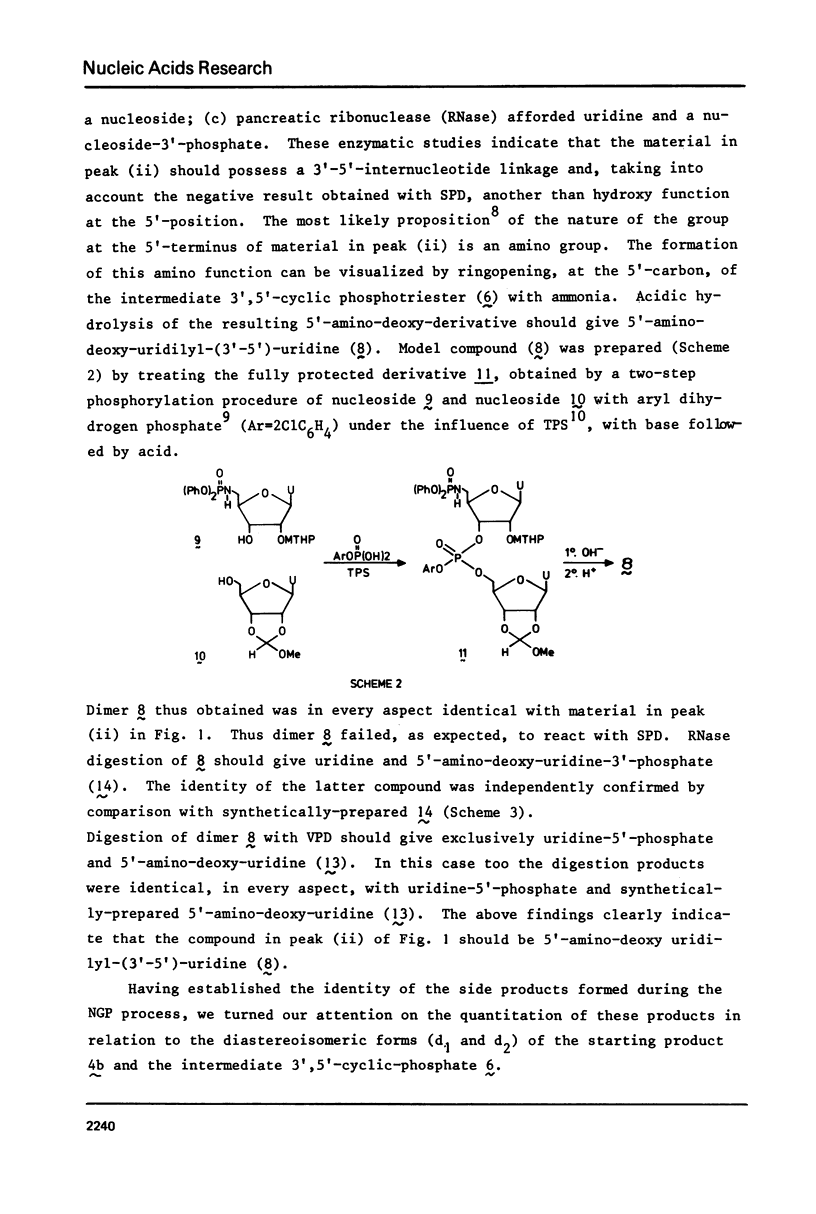
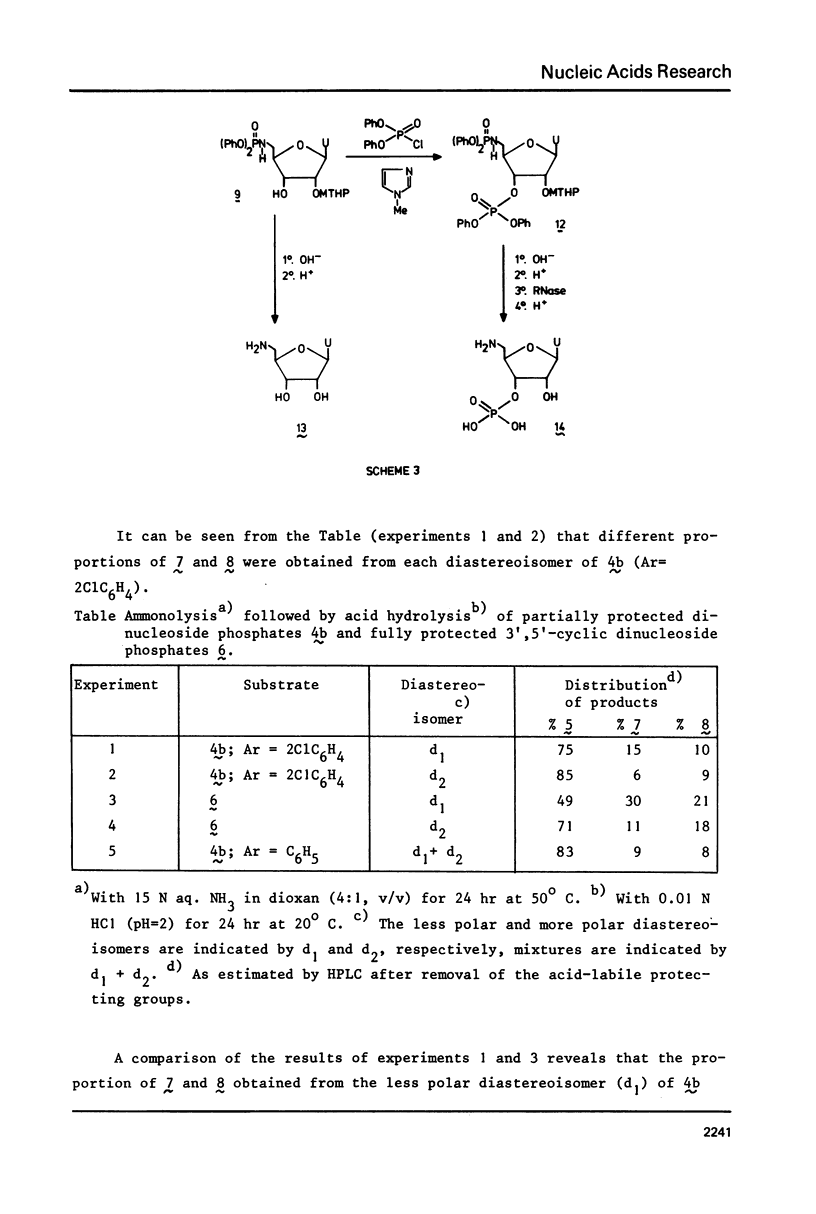
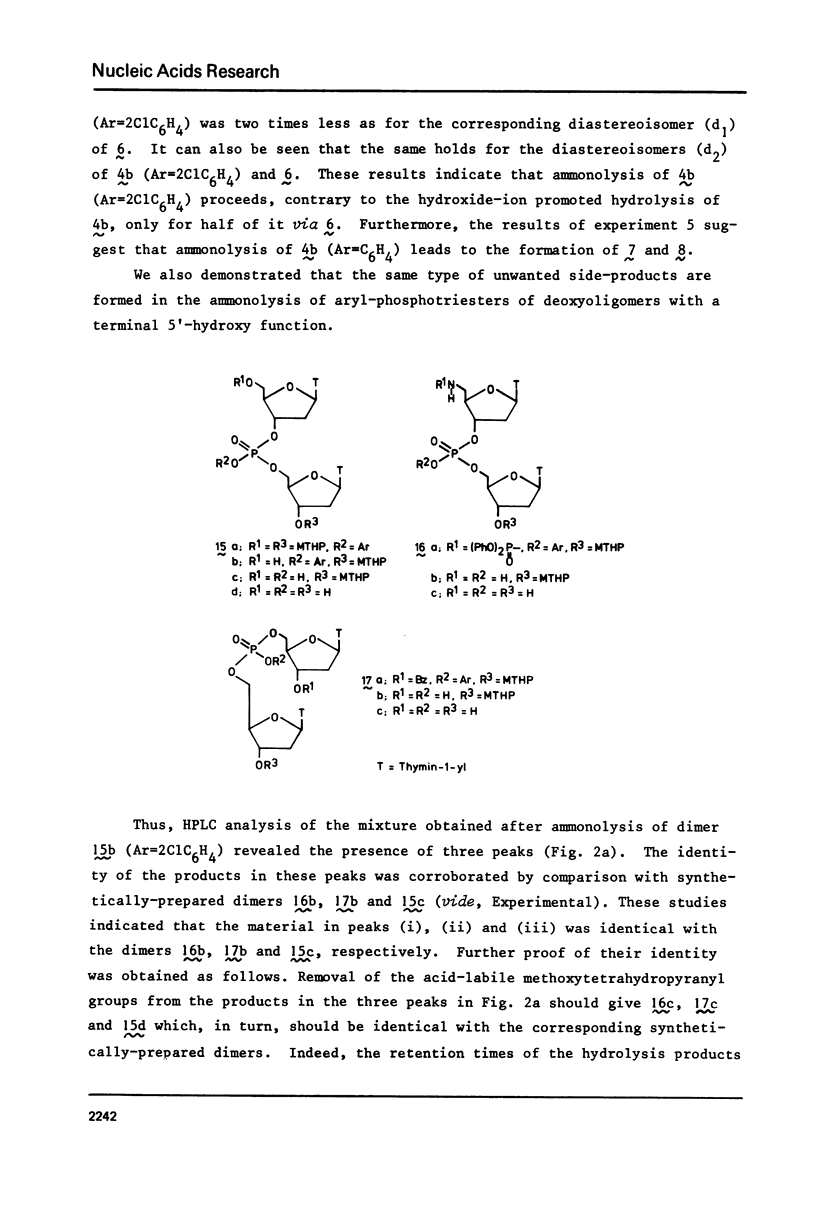
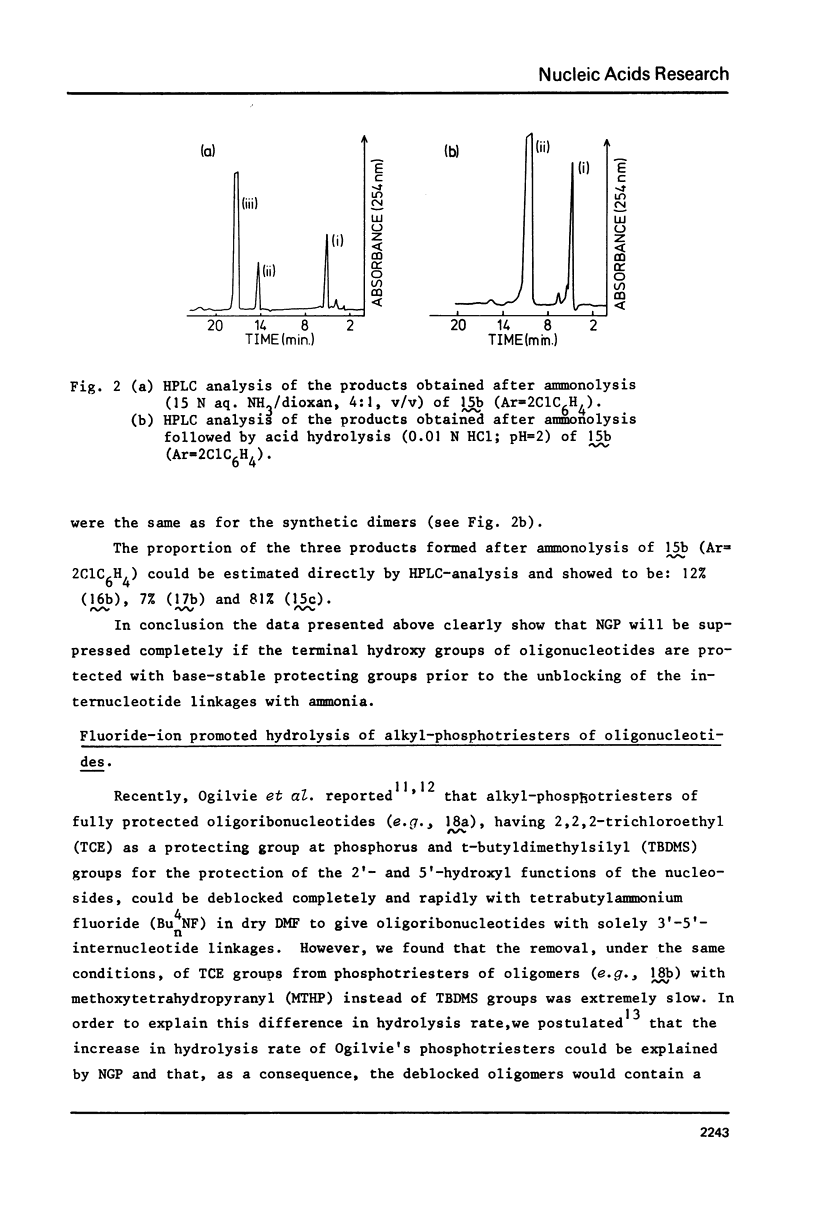
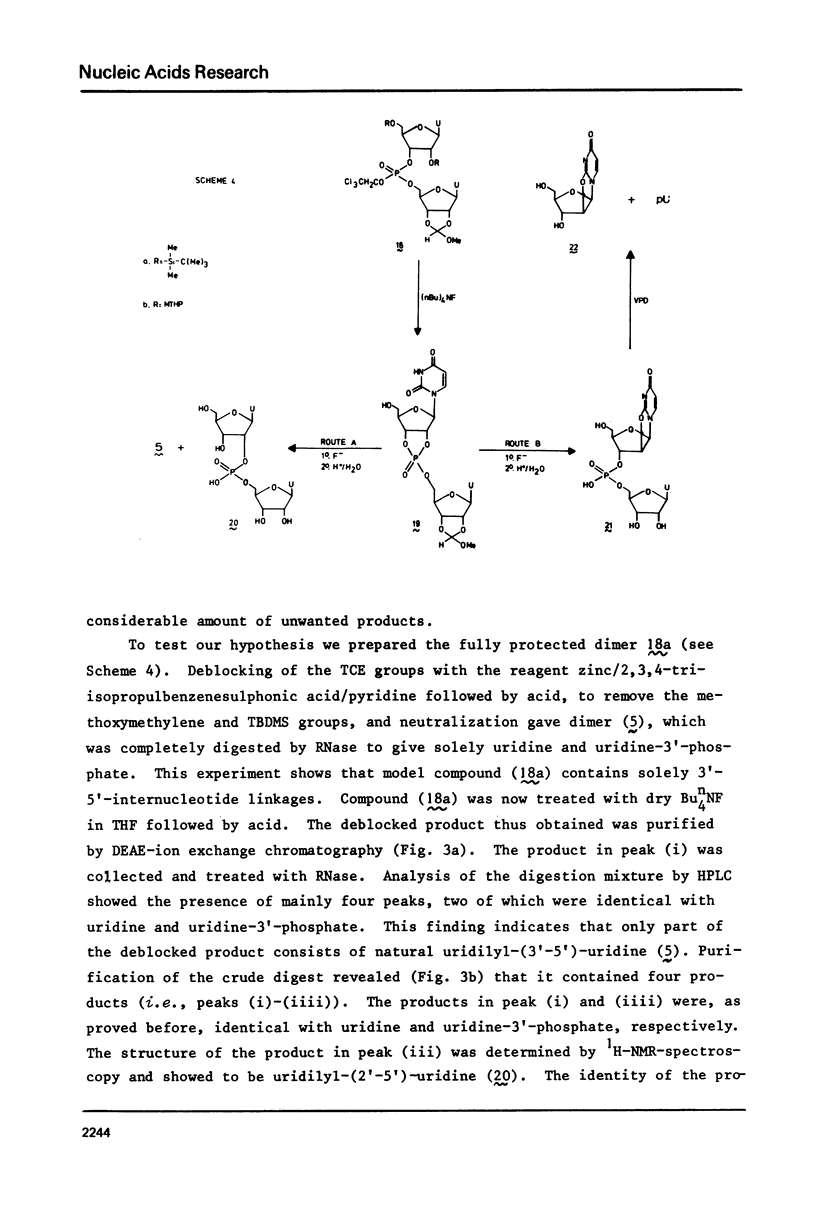
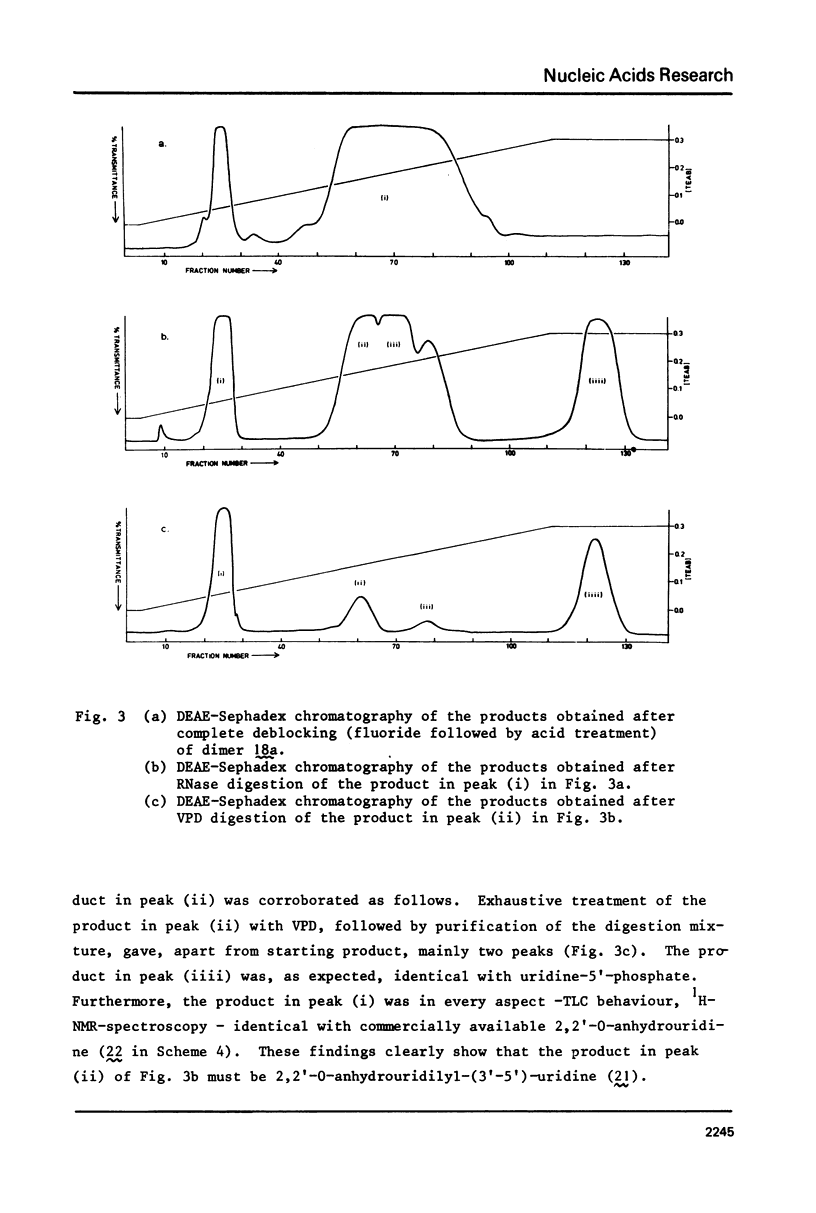
Two examples of neighbouring group participation during the removal of protecting groups from phosphotriesters of partially or fully protected intermediates of nucleic acids are presented. The first example shows that ammonolysis of aryl groups from phosphotriesters of partially protected - 5'- hydroxy free - nucleic acids (e.g., 4b approximately to; Ar=2C1C 6H4) gives rise to the formation of unnatural nucleic acids (e.g., 7 approximately to and 8 approximately to). The second one illustrates that fluoride ion promoted hydrolysis of 2,2,2-trichloroethyl groups from phosphotriesters of fully protected nucleic acids (e.g., 18a approximately to), having t-butyldimethylsilyl groups at the 2'-positions, leads to the formation of a considerable amount of side-products (e.g., 20 approximately to and 21 approximately to).
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arentzen R., Reese C. B. The phosphotriester approach to oligonucleotide synthesis: Preparation of oligo- and poly-thymidylic acids. J Chem Soc Perkin 1. 1977;4:445–460. doi: 10.1039/p19770000445. [DOI] [PubMed] [Google Scholar]
- Dalton J. G., George A. L., Hruska F. E., McGaig T. N., Ogilvie K. K., Pelling J., Wood D. J. Comparison of arabinose and ribose nucleoside conformation in aqueous and dimethylsulfoxide solution. 2',5'-Hydrogen bonding in arabinonucleosides. Biochim Biophys Acta. 1977 Oct 4;478(3):261–273. doi: 10.1016/0005-2787(77)90144-7. [DOI] [PubMed] [Google Scholar]
- Freist W., Schattka K., Cramer F., Jastorff B. Neue Darstellungsmethode von Nucleotid-Analogen der 5'-Amino-5'-desoxy-nucleoside. Chem Ber. 1972;105(3):991–999. doi: 10.1002/cber.19721050330. [DOI] [PubMed] [Google Scholar]
- Green D. P., Ravindranathan T., Reese C. B., Saffhill R. The synthesis of oligoribonucleotides-8. The preparation of ribonucleoside 2',5'-bisketals. Tetrahedron. 1970 Feb;26(4):1031–1041. doi: 10.1016/s0040-4020(01)98780-0. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B., Sulston J. E. The synthesis of oligoribonucleotides. II. Methoxymethylidene derivatives of ribonucleosides and 5'-ribonucleotides. Tetrahedron. 1967 May;23(5):2301–2313. doi: 10.1016/0040-4020(67)80067-x. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Ezra F. S., Kondo N. S., Sarma R. H., Danyluk S. S. Conformational properties of dinucleoside monophosphates in solution: dipurines and dipyrimidines. Biochemistry. 1976 Aug 10;15(16):3627–3639. doi: 10.1021/bi00661a034. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Sarma R. H. Aqueous solution conformation of rigid nucleosides and nucleotides. J Am Chem Soc. 1976 Jun 9;98(12):3541–3548. doi: 10.1021/ja00428a026. [DOI] [PubMed] [Google Scholar]
- Lohrmann R., Söll D., Hayatsu H., Ohtsuka E., Khorana H. G. Studies on polynucleotides. LI. Syntheses of the 64 possible ribotrinucleotides derived from the four major ribomononucleotides. J Am Chem Soc. 1966 Feb 20;88(4):819–829. doi: 10.1021/ja00956a039. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Theriault N., Sadana K. L. Synthesis of oligoribonucleotides. J Am Chem Soc. 1977 Nov 9;99(23):7741–7743. doi: 10.1021/ja00465a073. [DOI] [PubMed] [Google Scholar]
- Reese C. B., Saffhill R., Sulston J. E. 4-methoxytetrahydropyran-4-yl. A symmetrical alternative to the tetrahydropyranyl protecting group. Tetrahedron. 1970 Feb;26(4):1023–1030. doi: 10.1016/s0040-4020(01)98779-4. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., Burgers P. M., van der Marel G., Verdegaal C. H., Wille G. Synthesis of oligonucleotides with sequences identical with or analogous to the 3'-end of 16S ribosomal RNA of Escherichia coli: preparation of A-C-C-U-C-C via the modified phosphotriester method. Nucleic Acids Res. 1977 Apr;4(4):1047–1063. doi: 10.1093/nar/4.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boom J. H., de Rooy J. F., Reese C. B. The synthesis of oligoribonucleotides. X. Preparation of 2',3'-cyclic phosphates of ribonucleosides and diribonucleoside phosphates via phosphotriester intermediates. J Chem Soc Perkin 1. 1973;21:2513–2517. doi: 10.1039/p19730002513. [DOI] [PubMed] [Google Scholar]


