Abstract
Monocytes play an important role in hemostasis. In this study, the prothrombotic effects of the neuropeptide substance P (SP) on human monocytes through neurokinin-1 receptor (NK1-R) were characterized. SP upregulated monocyte tissue factor (TF), the major coagulation cascade stimulator, in a concentration and time dependent manner. Specific inhibition of NK1-R completely blocked TF expression. Monocytes stimulated by SP released cytokines and chemokines. When monocytes were stimulated with cytokines or chemokines, TF was expressed by the cytokines (GM-CSF, IFN-γ and TNF-α). Cytokines may play a major role in the mechanism of SP induced monocyte TF expression. NK1-R antagonists (NK1-RA) may have a role in developing novel therapeutic approaches to patients vulnerable to vaso-occlusive disorders.
Keywords: Substance P, Neurokinin receptors, Tachykinin, Thrombosis, Cytokines, Tissue factor
1. Introduction
Plasma levels of the neuropeptide SP (Michaels et al., 1998; Douglas et al., 2011; Douglas et al., 2001; Douglas et al., 2008; Fundeburg et al., 2010) and TF (Key et al., 1998; Michaels et al.,1998; Fundeburg et al., 2010) are elevated in patients with vaso-occlusive disorders including sickle cell disease (SCD), human immunodeficiency virus (HIV) infection, and other conditions (Douglas et al., 2011). A number of newly identified regulatory molecules including gas6 (Hurtado et al., 2010), CD40L (Andre et al.,2002), semaphorin 4D (Zhu et al., 2007), semaphorin 3A (Kashiwagi et al., 2005) and ephrins/eph kinases (Prevost et al., 2002) were discovered and have a role in the mechanism of thrombus formation. SP treatment stimulates activation and aggregation of platelets and platelets contain SP immunoreactivity that is released upon activation (Jones et al., 2008). In this study we demonstrate that SP, a member of the tachykinin family, may also contribute to thrombus formation by stimulating the expression of TF on monocytes.
SP is an important neuroimmune stimulator of innate immune functions of monocytes/macrophages (Bremer et al., 2010), released from the nucleus of the solitary tract in the brainstem and other central nervous system (CNS) sites (Bost et al., 2004). In addition, it is a potent pro-inflammatory mediator which plays an important role in inflammation and HIV infection (Michaels et al., 1998). SP mediates the biologic responses through an interaction with the NK1-R, a G-protein coupled receptor characterized by seven transmembrane domains (Lucey et al., 1994). NK1-R is present on immune cells (Khawaja et al., 1996; Ho et al., 1997) such as monocytes/macrophages (Ho et al., 1997; Lucey et al., 1994), neutrophils (Wozniak et al., 1989), T and B lymphocytes (Lai et al., 1998, Payan et al., 1984, Stanisz et al., 1987), and mast cells (Shanahan et al., 1985). There are two isoforms of NK1-R: full length NK1-R (NK1-RF) composed of 407 amino acids (aa), and a naturally occurring splice variant with a truncated C-terminus which is designated truncated NK1-R (NK1-RT) (Satake et al., 2006; Fong et al., 1992; Zhang et al., 2006), which has a 311 aa sequence with a short carboxyl terminal sequence, extending only 7 aa residues after the end of the seventh transmembrane segment (Tulac et al., 2009; Lai et al., 2006). The remaining aa sequence of the NK1-RT isoform is identical to that of NK1-R-F21 (Fong et al., 1992). This interaction occurs in several cell systems and is involved in exocrine gland secretion, endocrine secretion, pain transmission, vasodilatation, connective tissue cell proliferation, and neuroimmune modulation (Bost et al., 2004; Satake et al., 2006). The NK1-RT probably mediates prolonged cellular responsiveness after stimulation and is resistant to homologous desensitization in comparison to the NK1-RF isoform because the missing C-terminal domain is essential for internalization, receptor desensitization and recycling (Tulac et al., 2009). NK1-RA are a novel therapeutic approach to stress, mood therapy, and control of emesis (Douglas et al., 2011; Blume et al., 2011). NK1-RA (n-acetyl-L-tryptophan), significantly reduced edema formation and blood-brain barrier (BBB) permeability at 24 hours post-ischemia and significantly improved functional outcome as assessed over 7 days when administered 4 hours after onset of ischemic stroke in an animal model (Turner et al., 2011).
Tissue factor, also known as factor III, thrombokinase, or CD142, is a protein present in sub-endothelial tissue, platelets, and leukocytes that is necessary for the initiation of thrombin formation from the zymogen prothrombin. Full-length Tissue Factor (flTF), a 47 kDa transmembrane glycoprotein, is encoded by a mature mRNA transcript that consists of six exons: exon 1 encodes the N-terminal signal sequence, exons 2–5 encode the extracellular domain (aa 1–219), exon 6 encodes the transmembrane region (aa 220–244) and cytoplasmic tail (aa 245–263) (van den Berg et al., 2010). TF enables cells to initiate the blood coagulation cascades, and it functions as the high-affinity receptor for the coagulation factor VII. In addition, TF promotes the formation of factor VIIa, which converts factor X to factor Xa, which cleaves prothrombin to thrombin. Normally, TF is detectable in the adventitial cells within the vessel wall only (Drake et al., 1993). TF may be totally encrypted, which would allow circulating TF to be present without leading to generalized coagulation.TF, however can be induced by endotoxin on the cell surface of the endothelial cell, monocyte, platelet or the mononuclear cell by cleaved high molecular weight Kininogen (HKa) (Khan et al., 2010).
Mononuclear phagocytes are key inflammatory components of the cellular immune response; they are involved in cytokine production which include IL-1, IL-6, TNF-α and IL-12 (Michaels et al., 1998; Douglas et al., 2011; Khan et al., 2006). Cytokines interact with all phases of the immune system (Douglas et al., 2011) and are known stimulators for TF expression in monocytes (Khan et al., 2010). Cytokines enhance adhesion of leukocytes to endothelium and may play a role in vaso-occlusive events (Michaels et al.,1998). SP is also a known stimulator of TNF-α release and a promoter of IL-8. Plasma SP is increased in SCD (Michaels et al.,1998). SP and its preferred receptor, NK-1R, are central mediators in the interaction between the immune system and the nervous system (Douglas et al., 2001).
Involvement of neuropeptides in the pathophysiology of thrombus formation has not been characterized. We have investigated the effects of SP on the stimulation of human monocyte TF expression through NK1-R. This pathway may be a new stimulator for the mechanism(s) of thrombus formation in vaso-occlusive disorders.
2. Materials and Methods
2.1. Human monocytes
Human peripheral blood monocytes were obtained from healthy adult donors (n=78) from the Penn Center for AIDS Research Immunology Core (Philadelphia, PA, USA). Monocytes were purified from Apheresis products by using RosetteSep Human Monocyte kits purchased from STEMCELL Technologies Inc. (Vancouver, BC, Canada). The cells were suspended in Hanks’ balanced salt solution (HBSS), 0.1% Human Serum Albumin (HSA). These cell populations were >97% monocytes, as determined by nonspecific esterase staining and fluorescence-activated cell sorting (FACS) analysis using monoclonal antibodies (MAB) against CD14 (Leu-M3) and low-density lipoprotein specific for monocytes. Before activation with SP, the cell population was checked for activation status at the time of preparation by using CD69, CD16 (BD Biosciences, San Jose, CA.), CD142 (anti-Human TF, American Diagnostica, Stamford, CT.) of the CD45+CD14+ (Monocyte) population using a BD LSRII flow cytometer and FACS Diva acquisition and analysis software (BD Biosciences, San Jose, CA). The activated population (positive) was measured as a percentage of positive target cell events above the isotype (negative) control signal. Activated cell populations were <0.7%.
2.2. Proteins and antibodies
SP was purchased from Sigma (St. Louis, MO). The NK1-RA, aprepitant (Emend®), manufactured by Merck, was purchased through the Children's Hospital of Philadelphia Pharmacy and purified by chromatography (Chernova et al., 2009). The nonpeptide SP antagonist, CP-96345, and its 2R, 3R inactive enantiomer CP-96,344 were used as previously described (Lai et al., 2006). Recombinant human cytokines: GM-CSF, IFNγ, TNF-α; chemokines: MIP-1α, MIP-1β, RANTES; and neutralizing MAB against GMCSF, IFNγ, TNF-α and corresponding isotype controls either IgG2a or IgG1 were purchased from R & D Systems (Minneapolis, MN). Imubind Tissue Factor ELISA kit was purchased from American Diagnostica (Stamford, CT).
2.3. Control of lipopolysaccharide (LPS) contamination
Sterile and pyrogen-free working conditions were maintained to minimize any contamination by LPS. LPS assayed using QCL-1000 Chromogenic Limulus Amebocyte lysate kit from BioWhittaker (Walkersville, MD) indicated that all proteins and reagents had <0.01 EU/ml LPS.
2.4. Flow cytometric analysis and ELISA for TF expression
LPS-free SP (0.001, 0.01, 0.1, 1.0, 5.0 and 10.0 µM) was incubated for 4 hours at 37° C with 2 × 106/ml monocytes. Separately, LPS-free SP (5 µM) was incubated for 0, 30, 60, 120, 180, 240 and 360 minutes at 37° C with 2 × 106/ml monocytes suspended in HBSS. Following this incubation, the cell suspension was centrifuged at 13,000g for 5 minutes and the supernatant was used to assay cytokine production using BD Cytometric Bead Array (BD CBA) Flex kits: GM-CSF, IFN-γ, TNF-α, MIP-1α, MIP-1β, and RANTES (BD Biosciences, San Jose, CA). Each capture bead in the array has a unique fluorescence intensity (APC vs. APC-Cy7) and is coated with a capture antibody specific for a single analyte. A combination of the selected beads is mixed with the supernatant and a mixture of detection antibodies that are conjugated to a reporter molecule PE to form sandwich complexes. Following incubation and subsequent washing, the samples were acquired on a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). Each bead’s detection antibody MFI was measured on the PE channel of the flow cytometer. Quantitative cytokine levels (pg/mL) of each supernatant were determined from a standard curve generated using FCAP Array software(BD Biosciences, San Jose, CA). The pellet was re-suspended in 1% Triton X-100 in 0.05 M Tris and 0.1M NaCl (pH 8.5) and stirred for 12 hours at 4°C. The suspension was centrifuged at 13,000 g for 5 minutes to separate cell debris. TF antigen levels were measured in supernatant using the Imubind Tissue Factor ELISA kit (American Diagnostica, Greenwich, CT).
2.5. Thrombin generation assay (TGA)
Thrombin generation was measured in platelet poor plasma according to previously described methods with some modifications (Hemker et al., 2003). Severe hemophilia A plasma (< 1% FVIII activity; George King Biomed) was used in order to analyze TF-dependent thrombin generation in these assays. Forty µL of plasma was mixed in a black microtiter plate with 10 µL of either SP-stimulated cell lysate or Innovin diluted in phospholipid vesicles (Phosphatidylcholine:PC; Phosphatidylserine:PS: 75:25 PC:PS, 4 µM). Fifty µL of Z-Gly-Gly-Arg-AMC (1 mM in 15 mM CaCl2) was added to initiate coagulation and thrombin generation was measured by monitoring fluorescence for 90 minutes at 37°C in a Spectramaz M2e (Molecular Devices) with excitation and emission wavelengths of 360nm and 460nm, respectively. Thrombin concentrations were calculated from the raw fluorescence data using a thrombin calibrator (Technothrombin TGA calibrator). Thrombograms (nM thrombin vs. time) were plotted and used to determine parameters including lag time, peak thrombin generation and ETP.
2.6. Receptors involved in the expression of TF from human monocytes by SP
Monocytes (2 × 106/ml) were pre-incubated with or without the NK1-RA dissolved in DMSO, aprepitant (0, 1, 5, or 10 µM), CP96345 (10 µM) or its inactive enantiomer, CP96344 (10 µM) for 30 min at 37° C before stimulation with SP (5 µM) for an additional 240 min at 37° C. Untreated monocytes served as controls. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and samples were assayed for TF by ELISA.
2.7. TF expression on monocytes by GM-CSF, IFNγ, TNF-α and chemokines MIP-1α, MIP-1β, RANTES
Using identical concentrations of chemokines and cytokines released from monocytes by SP, monocytes (2 × 106/ml) were incubated with recombinant GM-CSF (10 pg/ml), IFNγ (10 pg/ml), TNF-α (2 ng/ml) and chemokines MIP-1α (10 ng/ml), MIP-1α (60 ng/ml), RANTES (2 ng/ml) for 0, 30, 60, 120, 180, and 240 minutes at 37° C. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 min and samples were assayed for TF by ELISA.
2.8. TF expression on monocytes by SP (5 µM) in presence of blocking monoclonal antibodies to GM-CSF, IFNγ, TNF-α
Monocytes (2 × 106/ml) were incubated with or without MAB to GM-CSF, IFNγ, and TNF-α at the doses (10 fold excess) adjusted by calculating ND50 for corresponding cytokines. ND50 for GM-CSF is 0.16 µg/ml, for IFNγ is 0.06 µg/ml, and for TNF-α is 0.01µg/ml as recommended by the manufacturer (R & D Systems, Minneapolis, MN). MABs were incubated for 30 minutes at 37° C before stimulation with SP (50µM) for another 240 minutes at 37° C. Corresponding isotype IgG1/IgG2a and HSA or MAB to HSA served as controls. Following this incubation, the cell suspension was centrifuged at 13,000 g for 5 minutes and samples were assayed for TF by ELISA.
2.9 Statistical analysis
Each experiment was performed at least three separate times and in triplicate. Values displayed represent mean ± standard error of the mean. PNK1-R values were determined by Student’s t-test (between groups) or one-way analysis of variance (ANOVA) comparing more than two groups. Differences were considered significant when P<0.05.
3. Results
3.1. SP up-regulated TF expression on monocytes in a concentration dependent manner
Following incubation with SP, monocytes were incubated with Triton-X-100 overnight for complete cell lysis to obtain flTF. We used an ELISA to measure flTF antigen on monocytes. SP up-regulated TF expression in a concentration-dependent manner. For 1.0 µM of SP, levels of TF were 151.97 ± 54.6 pg/ml (n=5). Similarly, for 5.0 µM of SP, TF levels were 485.63 ± 72.55 pg/ml (n=16) and for 10.0 µM of SP, TF levels were 160.84 ± 89.72 pg/ml (n=5) (Figure 1A). At concentrations below 0.1 µM, SP did not stimulate TF expression. As a positive control, LPS (10 ng/ml) increased TF expression to 1883.48 ± 373.3 pg/ml (n=5). Untreated cells (control) expressed TF to 62.71± 18.74 pg/ml (n=16).
Figure 1. Effect of SP on monocyte TF expression.
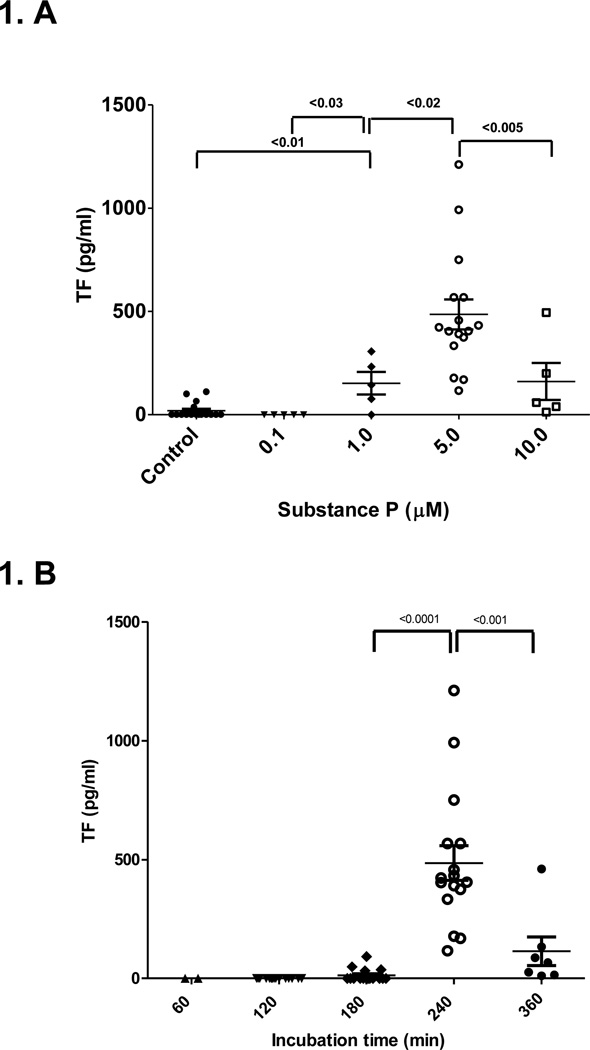
A. Monocytes were incubated for 240 minutes at 37 °C with different concentrations of SP as indicated. Untreated monocytes were used as a control. SP induced monocyte TF expression was determined at SP concentrations of 1.0 µM (♦, n=5), 5.0 µM (○ n=16) and 10.0 µM (□, n=5). Median values (horizontal lines) and significance are shown as indicated.
B. Monocytes were incubated with SP (5.0 µM) at 37 °C for different time points as indicated. Untreated monocytes were used as a control. SP induced monocyte TF expression was determined from 0, 60 (▲, n=3), 120 (♣, n=8), 180 (♦, n=16), 240 (○ n=16) and 360(●, n=7) minutes. Median values (horizontal lines) and significance are shown as indicated.
3.2. SP up-regulated TF expression on monocytes in a time dependent manner
Monocytes (2 × 106/ml) were stimulated by SP (5 µM) for 0, 30, 60, 120, 180, 240 and 360 minutes at 37°C (n=16). TF expression was first detected at 3 hours at 13.21 ± 6.64 pg/ml and reached a maximum response of 485.63 ± 72.55 pg/ml at 4 hours. At 6 hours, TF expression was 114.09 ± 60.26 pg/ml (Figure 1B).
3.3. Assessment of thrombin generation by SP induced monocyte TF
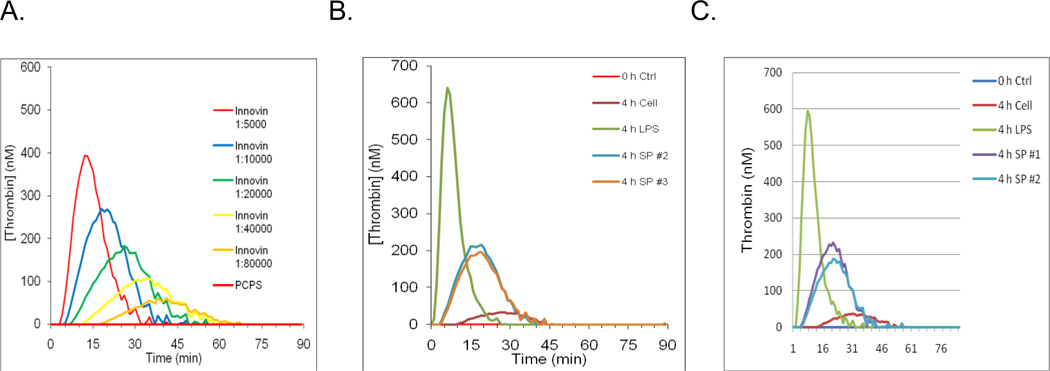
In order to determine whether the TF expressed on SP stimulation to monocytes is active, monocytes (2 × 106/ml) were stimulated by SP (5 µM) for 240 minutes at 37° C (n=4) and applied to TGA (Bunce et al., 2011) after three cycles of freezing on dry ice followed by thawing at 37° C to completely lyse all cellular elements according to methods described previously (Key et al., 1998). In order to specifically monitor TF-dependent thrombin generation and eliminate thrombin generation by the intrinsic coagulation pathway, TGAs were performed in FVIII-deficient plasma. As a control for TF activity, serial dilutions of Innovin were used to initiate thrombin generation. Innovin affected thrombin generation in a dose-dependent manner, with increasing Innovin concentrations leading to greater thrombin generation and shorter lag times (Figure 2 A). LPS-stimulated cell lysates induced a strong thrombin generation profile with peak thrombin concentration of 640 nM (4 h LPS, green, Figure 2B) while unstimulated cells produced only a peak of 32 nM thrombin and displayed a prolonged lag time (4 h Cell, magenta, Figure 2B). SP-treated cells displayed appreciable TF activity in these assays, with 4 of 5 replicates generating a peak of 200 nM thrombin (Figures 2B, 2C) comparable to a 1:20,000 dilution of Innovin. These results indicated that TF expressed by SP stimulation of monocytes is biologically active.
Figure 2.
Influence of the SP induced monocyte TF on thrombin generation in hemophilic plasma. Thrombin generation was measured for 90 min at 37 °C in hemophilia A plasma supplemented with either SP-stimulated cell lysate or Innovin diluted in phospholipid vesicles (Phosphatidylcholine:PC; Phosphatidylserine:PS: 75:25 PC:PS). Thrombin generation was initiated with CaCl2 and a thrombin fluorogenic substrate as described in “Methods”. As a control for TF activity, serial dilutions of Innovin were used to initiate thrombin generation (A). Figure 2B and 2C are two independent experiments. LPS stimulated cell lysates induced a strong thrombin generation profile (4 h LPS, green) while unstimulated cells displayed a prolonged lag time (4 h cell, red and magenta) (Fig 2 B, C). SP-treated cells displayed TF activity (4 h SP #2 and 4 h SP# 3, blue and orange) (B) and (4 h SP #1 and 4 h SP #2, purple and blue (C). In these assays generating a peak of 200 nM thrombin comparable to a 1:20,000 dilution of Innovin.
3.4. CP96,345 (SP inhibitor) or Aprepitant (NK1-RA) Inhibited TF expression on monocytes
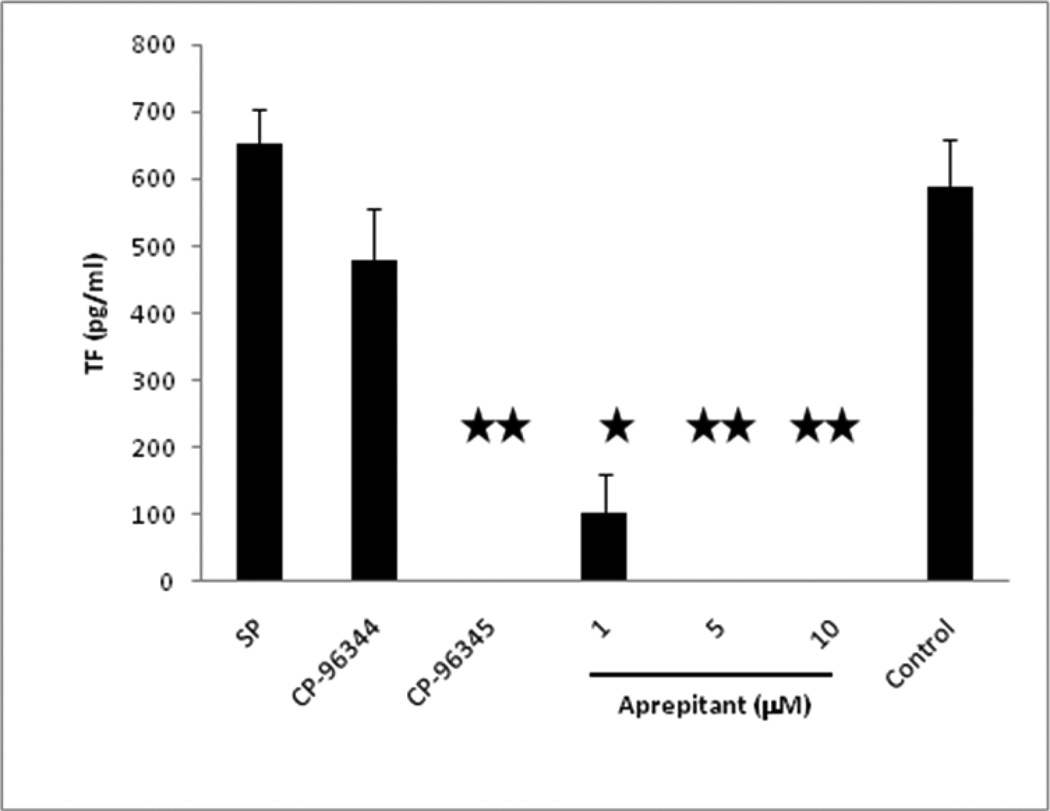
In order to determine whether inhibition of CP-96,345 on SP (Lai et al., 2001) induced monocyte TF expression is mediated by specific antagonism of NK1-R, monocytes were incubated with CP-96,345 or its inactive enantiomer CP-96,344 for 30 minutes and then stimulated with SP. CP96,345 (10 µM) had completely inhibited SP (5 µM) stimulated TF expression (651.44 ± 53.21 pg/ml) on monocytes (n=3) whereas CP-96,344 at the same concentration had minimal inhibitory effect (Figure. 3), demonstrating that CP-96,345 affected SP induced monocyte TF expression through antagonism of NK1-R on monocytes.
Figure 3. Effect of aprepitant and CP-96,345 on SP induced monocyte TF expression.
Monocytes were incubated in presence or absence of aprepitant, CP-96,345 or CP-96,344 as indicated. CP-96,344 was used as a specificity control. The data shown are the mean ± SEM of triplicate and are representative of three experiments. (* p<0.001; **, p<0.01, aprepitant and CP-96,345 treated vs. SP).
A similar effect was observed with SP-antagonist aprepitant (5 and 10 µM), which had completely inhibited SP (5 µM) stimulated TF expression on monocytes (n=3) (Figure 3). Aprepitant (1µM) partially inhibited SP stimulated TF expression (651.44 ± 53.21 pg/ml to 102.0 ± 58.11 pg/ml). As a control, dimethyl sulfoxide (DMSO) or CP 96,344 (10 µM), failed to inhibit SP stimulated TF expression. In the presence of DMSO and CP 96,344 (10 µM), SP stimulated TF expression was (587.33 ± 70.77 pg/ml) and (478.33 ± 78.80 pg/ml), respectively. These data indicate that the SP stimulated TF expression is a result of activation of the NK1-R by Substance P.
3.5. SP Releases Cytokines and Chemokines From monocytes
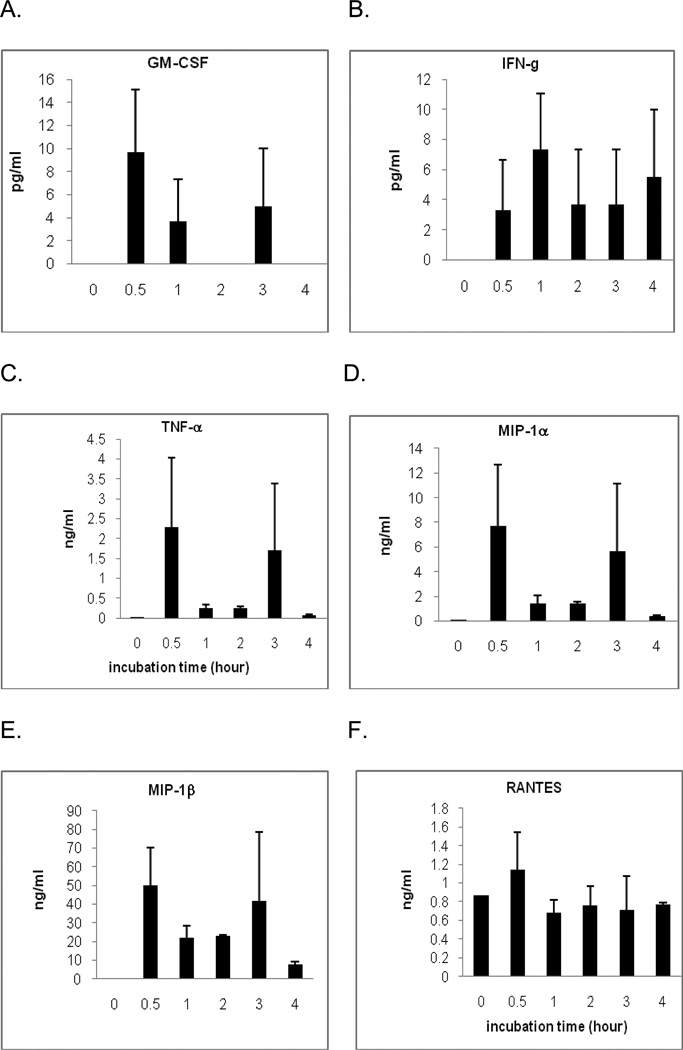
SP stimulates release of several cytokines and chemokines (Chernova et al. 2009) from monocytes. We have tested three from each group by stimulating monocytes (2 × 106/ml) with SP (5 µM) for 0, 30, 60, 120, 180, and 240 minutes at 37° C (n=3). In Figure 4, the release of cytokines is demonstrated. After 30 minutes, GM-CSF 9.06 ± 5.06 pg/ml (Figure 4A), IFN-γ 3.29 ± 3.51 pg/ml (Figure 4B), TNF-α 2.19 ± 1.49 ng/ml (Figure 4C). The release of chemokines is demonstrated as well. After 30 minutes, concentration of MIP-1α is 7.21 ± 3.56 ng/ml (Figure 4D), concentration of MIP-1β is 48.16 ± 11.63 ng/ml (Figure 4E), concentration of RANTES is 1.2 ± 0.36 ng/ml (Figure 4F).
Figure 4. Release of cytokines and chemokines from monocytes by SP.
SP was incubated for 0, 30, 60, 120, 180, and 240 minutes at 37° C with 2 × 106/ml monocytes. Cytokines [GM-CSF (A), IFN-γ (B), TNF- α (C)] and chemokines[MIP-1α (D), MIP-1β (E), RANTES (F)] are from monocytes as indicated. The data shown are presented as the mean ± SEM of triplicate and are representative of three experiments.
3.6. TF expression on monocytes by cytokines and chemokines
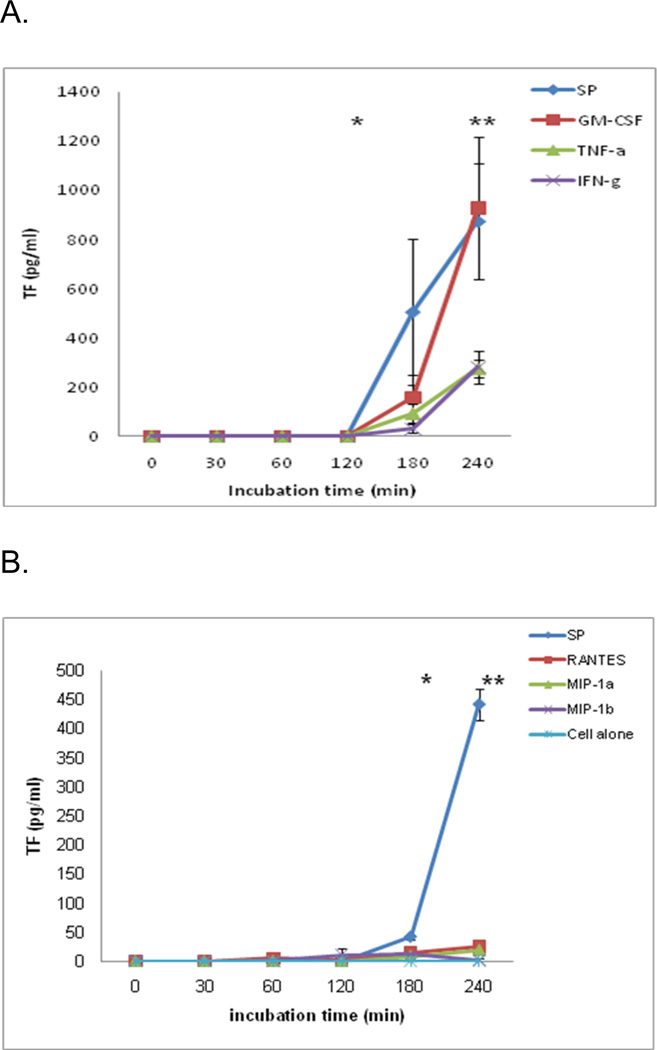
Using a concentration of each of the cytokines GM-CSF, IFN-γ and TNF-α that was released from monocytes one-half hour after stimulation by SP, we found that each of the cytokines had stimulated monocytes to express TF (Figure 5A) (n=3). GM-CSF expressed TF 158.72 ± 89.19 pg/ml at 3 hours and increased significantly to 927.64 ± 289.03 pg/ml at 4 hours (p< 0.05). IFN-γ expressed TF 30.25 ± 17.34 pg/ml at 3 hours and increased significantly to 280.96 ± 65.18 pg/ml at 4 hours (p<0.05). TNF-α expressed TF 93.11 ± 36.88 pg/ml at 3 hours and increased significantly to 275.03 ± 37.62 at 4 hours (p< 0.05). In this set of experiments, SP expressed TF 506.1 ± 295.9 pg/ml at 3 hours and increased significantly to 875.23 ± 235.34 pg/ml at 4 hours (p< 0.001).
Figure 5. Effect of cytokines and chemokines on monocyte TF expression.
A. Maximum cytokine concentration that was released from monocytes by SP, using same concentrations, monocytes were stimulated by GM-CSF (9.06 pg/ml), IFN-γ (7.69 pg/ml) and TNF-α (2.19 ng/ml). Each of the cytokines had stimulated monocyte to express TF as indicated. The data shown are the mean ± SEM of triplicate and are representative of three experiments. * p<0.05 represents 2 vs 3 h and 3 vs. 4 h of SP; **, p<0.001 represents GM-CSF, IFNγ and TNFα treated vs. SP at 4h and not significant (none) as indicated.
B. Maximum chemokine concentration that was released from monocytes by SP, using same concentrations, monocytes were stimulated by chemokines: MIP-1α (7.21ng/ml), MIP-1α (48.16 ng/ml), and RANTES (1.2 ng/ml). Each of the chemokines had stimulated monocyte to express TF as indicated. The data are the mean ± SEM of triplicate and are representative of three experiments.
* p<0.01 represents 3 h vs. 4 h of SP; **, p<0.001 represents MIP-1α, MIP-1β and RANTES treated vs. SP at 4h and not significant (none) as indicated.
Activation with chemokines did not lead to expression of TF significantly on monocytes (n=3) (Figure 5B). MIP-1α expressed TF 8.36 ± 8.21 pg/ml and 19.42 ± 8.24 pg/ml at 3 hours and 4 hours respectively. MIP-1β expressed TF 0.75 ± 0.75 pg/ml, 10.6 ± 10.62 pg/ml, 11.09 ± 4.06 pg/ml and 1.81 ± 1.82 pg/ml at 1, 2, 3 and 4 hours respectively. RANTES expressed TF 4.81± 3.58 pg/ml, 0.27 ± 0.27 pg/ml, 15.12 ± 8.21 pg/ml and 24.85 ± 8.24 pg/ml at 1, 2, 3 and 4 hours respectively. There were no significant differences between or within the groups in different time points. In this set of experiments, SP expressed TF at a concentration of 41.39 ± 4.08 pg/ml at 3 hours but the concentration of TF increased significantly to 441.09 ± 26.09 pg/ml at 4 hours (p< 0.001).
3.7. TF expression on monocytes by SP in presence or absence of MAB to GM-CSF, IFN-γ and TNF-α
Since the cytokines GM-CSF, IFN-γ, and TNF-α have the ability to express TF much like SP, we have further investigated the effect of SP on expression of TF in the presence or absence of NK1-R MAB to GM-CSF, IFN-γ and TNF-α. A MAB to TNF-α blocked SP induced TF expression from 226.45 ± 60.02 pg/ml to 43.52 ± 5.03 pg/ml (n=3) (Figure 6). In this set of experiments, expression of TF with SP alone was found to be 226.45 ± 60.02 pg/ml. The expression of SP with an isotype control (IgG1) for MAB to GM-CSF and TNF-α expressed TF was 90.04 ± 35.6 pg/ml. IgG2A for MAB to IFN-γ alone expressed TF at a concentration of 0.68 ± 0.56 pg/ml.
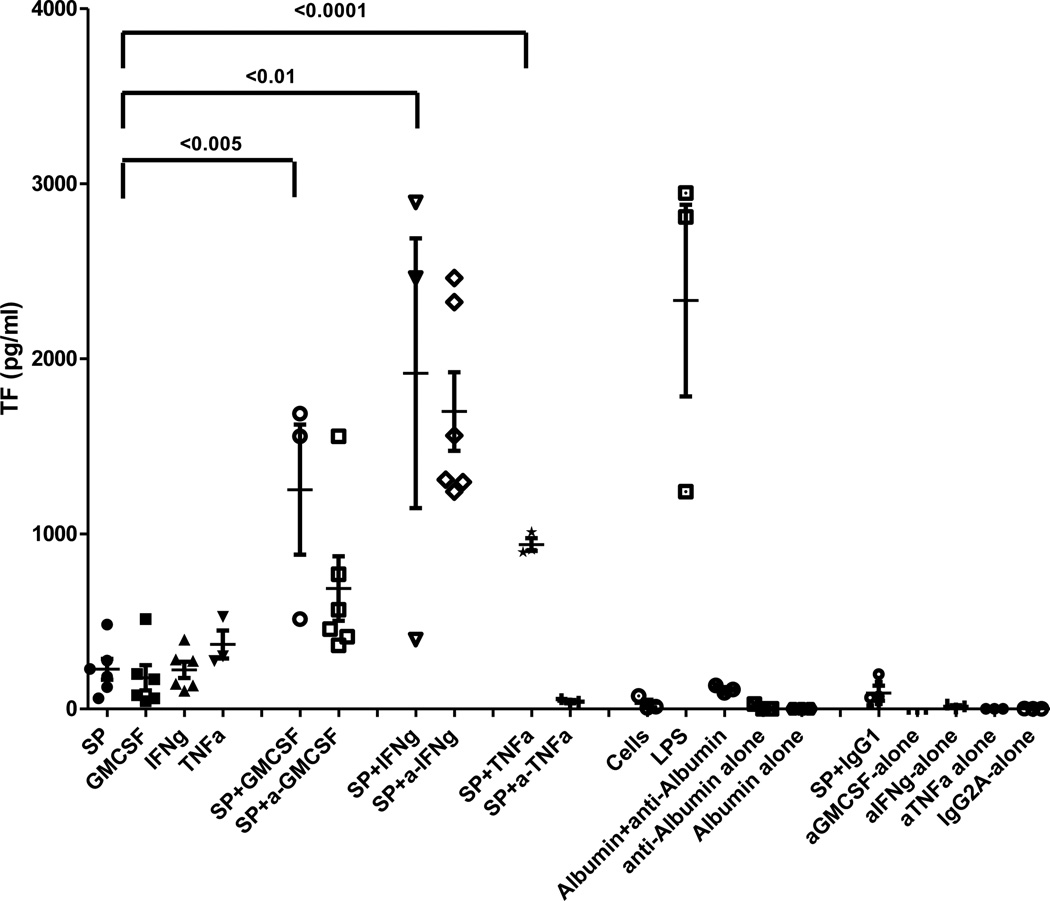
Figure 6. Effect of blocking MAB to GM-CSF, IFNγ, TNF- α on SP induced monocyte TF expression.
In order to determine if the effect of SP on monocyte TF expression was direct or indirect through the cytokine pathway, monocytes were stimulated with SP in the presence or absence of blocking MAB to GM-CSF, IFN γ, and TNF Effects of SP and GM-CSF, IFN-γ and TNF-α alone, with all controls are shown. The data are the mean ± SEM of triplicate and are representative of three experiments. p<0.005, 0.0001 and 0.01 represents significant as indicated.
In contrast, in the presence of a MAB to GM-CSF and IFN-γ, SP induced TF expression on monocytes was synergistically elevated to 687.44 ± 184.51 pg/ml from 226.45 ± 60.02 pg/ml (p<0.005) and 1698 ± 225.43 pg/ml from 226.45 ± 60.02 pg/ml respectively (n=3) (Figure 6). In order to determine the possible effect of an immune complex, we used HSA (Lonza) and MAB to HSA (R &D systems) on monocyte TF expression. We have observed that the albumin-antialbumin complex also expresses monocyte TF (112.38 ± 10.10 pg/ml) (n=3). In order to solve the unknown synergistic effect by MAB to GM-CSF or IFN-γ, we have conducted additional control experiments in the presence of SP (5 µM) with GM-CSF, IFN-γ and TNF-α using a concentration of each cytokine that was released from monocytes by SP. All of these cytokines synergistically increased TF in the presence of SP. For GM-CSF, TF was elevated from 177.87 ± 72.18 pg/ml to 1252.70 ± 263.54 pg/ml; for IFN-γ, TF was elevated from 222.82 ± 46.52 pg/ml to 1916.93 ± 546.83 pg/ml and for TNF-α, TF was elevated from 368.26 ± 56.66 pg/ml to 939.18 ± 25.85 pg/ml.
These results showed that MAB to TNF-α inhibited TF expression when monocytes were stimulated with SP alone. The experiments suggest TNF-α had effects the SP induced TF expression mechanism. It is possible that other cytokines may have similar effects.
4. Discussion
A number of different types of non-neuronal cells including lymphocytes and monocytes possess SP and its specific NK1-R(Marriotte et al., 2001); however, the pathophysiological roles of SP in these cells remain to be established SP promotes platelet-dependent clot formation through NK1-R, in which leukocytes are involved (Azma et al., 2009). SP triggers functional responses in many cells of the immune system through NK1-R including inflammatory processes and immunologic responses, involving monocytes, macrophages, lymphocytes, microglia, natural killer cells, and precursors of immune cells (Douglas et al., 2011; Lai et al., 1998; Ho et al., 2002; Ho et al., 1997).
By stimulating monocytes with SP, we found that TF antigen was expressed (Figure 1A) and using TGA revealed it was active (Figure 2). The time course shows no increase at 2 hours, a minimal increase at 3 hours, a maximum increase at 4 hours, and no increase at 6 hours (Figure 1B). This pattern of responses is similar to the synthesis of TF observed following stimulation of monocytes with bacterial LPS over time.
Peripheral tachykinins and the NK1-R are required for thrombus formation (Jones et al, 2008). Moreover, blood obtained from the NK1-R–deficient mice produced markedly smaller thrombi with a NK1-R agonist compared with blood from control mice suggesting that physiologically, SP is a major NK1-R agonist responsible for amplifying thrombus formation through the NK1-R (Bozic et al.,1996). Two naturally occurring variants of the NK1-R mediate the effects of SP: a full length, NK1-RF (407 aa) and a truncated form NK1-RT that lacks 96 amino acid residues at the C-terminus (311 amino acid). We have detected the truncated NK1-R in the non-differentiated monocytic THP-1 cell line and in primary monocytes using RT–PCR. Both full length and truncated forms were detected in the THP-1 cells differentiated into macrophages using PMA (Lai et al., 2006). The full length variant is capable of signaling, whereas the truncated NK1-R primes the chemokine receptor CCR5 (Chernova et al., 2009). The potential inhibitory roles of SP antagonist CP-96345 have been described in our laboratory. CP-96345 inhibits HIV (R5 strains) replication in human monocyte-derived macrophages (MDM) in part through the CCR5 receptor as well as through downregulation of other pathways (Lai et al., 2001). The SP antagonist, CP-96345, but not the inactive enantiomer CP-96344 inhibits TF expression. NK1-R is required for SP-induced monocyte TF expression (Figure 3) which is most likely mediated through NK1-RT, the predominant isoform in monocytes (Douglas et al., 2011).
SP activates NF-κB, a factor involved in the control of cytokine expression, and stimulates human peripheral blood mononuclear cells (PBMC) to produce inflammatory cytokines including IL-1, IL-6, TNF-α and IL-12 in HIV-infected patients (Bremer et al., 2010; Douglas et al., 2011). Cytokines (GM-CSF, IFNγ, TNF-α) and chemokines (MIP-1α, MIP-1β, RANTES) inhibit HIV-1 by suppressing viral entry and replication (Douglas et al., 2011). The role of SP on the expression of TF by these agents is unknown. We demonstrate that GM-CSF, IFNγ, TNF-α, MIP-1α, MIP-1β, and RANTES are released from monocytes after stimulated with SP (Figures 4A–4F). We have observed biphasic responses for all of the cytokines (GM-CSF, IFN-γ, TNFα) and chemokines (MIP-1α and MIP-1β) except RANTES in SP stimulated monocytes similar to previous studies where IL-8 and IL-6 mRNA were observed in parainfluenza virus type-4 (PIV-4) infected NCI-H292 cells (Rogers et al., 2004). In an another study using human whole blood as an ex vivo model of local cytokine production, TNFα, IL-1α, IL-1β, IL-6, and IL-8 were first detected between 1 and 4 hours post –LPS stimulation, and reached plateau levels after 6 to 12 hours (Deforge et al., 1992).
In order to determine the effect of chemokines/cytokines on the expression of TF treated with identical concentrations of chemokines and cytokines (released from monocytes after stimulated with SP), we found that chemokines (MIP-1α, MIP-1β, RANTES) failed to express TF on monocytes (Figure 5B). In contrast, stimulation with each of the cytokines leads to TF expression on monocytes (Figure 5A).
The blocking of SP induced TF expression by MAB to TNF-α suggests an indirect autocrine pathway in monocytes that depends on TNF-α (Figure 6). A similar mechanism was observed on monocyte TF expression by HKa (Khan et al., 2010). In contrast, SP induced TF expression on monocytes was synergistically augmented even in the presence of MAB against cytokines GM-CSF and IFN-γ (Figure 6). SP induced TF expression is mediated by TNF-α and not by GM-CSF or IFN-γ. We observed a synergistic effect of each cytokine with SP but only a MAB to TNF-α inhibited TF expression. This finding indicates the possibility of a different receptor or signaling pathway through which GM-CSF and IFN-γ expresses monocyte TF.
In summary, we have demonstrated that SP triggers TF synthesis on monocytes in a time and concentration dependent manner. NK1-R is the most critical receptor for SP-induced TF expression. SP induced TF expression was mediated by TNFα in an autocrine pathway. NK1-R may be a new and potential therapeutic target in the treatment of vaso-occlusive disorders.
ACKNOWLEDGMENTS
Mathew W Bunce and Rodney M Camire of the Department of Pediatrics, Children's Hospital of Philadelphia Research Institute, University of Pennsylvania Medical School (Philadelphia, Pennsylvania) brilliantly performed the thrombin generation assay with data interpretation. Dr. Florin Tuluc contributed to critical discussions on the NK1-R and NK1-RA for this project. This work was supported by NIH Grants #1U01-MH090325 (“Anti-HIV Neuroimmunomodulatory Therapy with Neurokinin-1 Antagonists”), #PO1-MH076388 [“Neurokinin-1R (SP Receptor) Antagonists for HIV Therapy”], and #RO1-MH049981-15 (“Tachykinins, Mononuclear Phagocytes and HIV-1 Infection”) to SDD. This work was made possible through core services and support from the Penn Center for AIDS Research (CFAR), an NIH-funded program (P30 AI 045008). We are grateful to Joshua A. Taton for his help in preparing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andre P, Prasad KS, Denis CV, He M, Papalia JM, Hynes RO, D.R. Phillips DR, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin–dependent mechanism. Nat Med. 2002;8:247–252. doi: 10.1038/nm0302-247. [DOI] [PubMed] [Google Scholar]
- Azma T, Matsubara Y, Kinoshita H, Hidaka I, Shiraishi S, Nakao M, Kawamoto M, Yuge O, Yoshio Hatano. Prothrombotic roles of substance-P, neurokinin-1 receptors and leukocytes in the platelet-dependent clot formation in whole blood. Journal of thrombosis and thrombolysis. 2009;27(3):280–286. doi: 10.1007/s11239-008-0215-0. [DOI] [PubMed] [Google Scholar]
- Bost KL. Tachykinin-mediated modulation of the immune response. Front. Biosci. 2004;9:3331–3332. doi: 10.2741/1484. [DOI] [PubMed] [Google Scholar]
- Bozic CR, Lu B, Höpken UE, Gerard C, Gerard NP. Neurogenic amplification of immune complex inflammation. Science. 1996;20;273(5282):1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun. 2011;25(2):221–229. doi: 10.1016/j.bbi.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer AA, Leeman SE. Encyclopedia of Life Sciences. Chichester. UK: John Wiley & Sons, Ltd.; 2010. Substance P; pp. 1–16. [Google Scholar]
- Bunce MW, Toso R, Camire RM. Zymogen-like factor Xa variants restore thrombin generation and effectively bypass the intrinsic pathway in vitro. Blood. 2011;117(1):290–298. doi: 10.1182/blood-2010-08-300756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernova I, Lai JP, Li H, Schwartz L, Tuluc F, Korchak HM, Douglas SD, Kilpatrick LE. Substance P (SP) enhances CCL5-induced chemotaxis and intracellular signaling in human monocytes, which express the truncated neurokinin-1 receptor (NK1-R) J. Leukocyte Biol. 2009;85:154–164. doi: 10.1189/jlb.0408260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge LE, Kenney JS, Jones ML, Warren JS, Remick DG. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992;148(7):2133–2141. [PubMed] [Google Scholar]
- Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann N Y Acad Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SD, Ho WZ, Gettes DR, Cnaan A, Zhao H, Leserman J, Petitto JM, Golden RN, Evans DL. Elevated substance P levels in HIV infected men. AIDS. 2001;15:2043–2045. doi: 10.1097/00002030-200110190-00019. [DOI] [PubMed] [Google Scholar]
- Douglas SD, Cnaan A, Lynch KG, Benton T, Zhao H, Gettes DR, Evans DL. Elevated substance P levels in HIV infected women in comparison to HIV-negative women. AIDS Res. Hum. Retroviruses. 2008;24:375–378. doi: 10.1089/aid.2007.0207. [DOI] [PubMed] [Google Scholar]
- Drake TA, Cheng J, Chang A, Taylor FB., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1458–1470. [PMC free article] [PubMed] [Google Scholar]
- Fundeburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, Luciano AA, Stevens W, Rodriguez B, Brenchley JM, Douek DC, Lederman MM. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115:161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TM, Anderson SA, Yu H, Huang RR, Strader CD. Differential activation of intracellular effector by two isoforms of human neurokinin-1 receptor. Mol. Pharmacol. 1992;41:24–30. [PubMed] [Google Scholar]
- Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Béguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33(1):4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- Ho WZ, Lai JP, Li Y, Douglas SD. HIV enhances substance P expression in human immune cells. FASEB J. 2002;16(6):616–618. doi: 10.1096/fj.01-0655fje. [DOI] [PubMed] [Google Scholar]
- Hurtado B, de Frutos PG. GAS6 in systemic inflammatory diseases: with and without infection. Crit Care. 2010;14(5):1003. doi: 10.1186/cc9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Tucker KL, Sage T, William J, Kaiser WJ, Barrett NE, Lowry PJ, Zimmer A, Hunt SP, Emerson M, Gibbins JM. Peripheral tachykinins and the neurokinin receptor NK1 are required for platelet thrombus formation. Blood. 2008;111(2):605–612. doi: 10.1182/blood-2007-07-103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106:913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- Khan MMH, Liu Y, Khan ME, Gilman ML, Khan ST, Bromberg ME, Colman RW. Upregulation of tissue factor in monocytes by cleaved high molecular weight kininogen is dependent on TNFα and IL-1β. Am J Physiol (Heart Circ Physiol.) 2010;298:H652–H658. doi: 10.1152/ajpheart.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int. J. Biochem. Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- Khan MM, Bradford HN, Isordia-Salas I, Yuchuan Liu, Yi Wu, Espinola RG, Ghebrehiwet B, Colman RW. High molecular weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1 and gC1qR in monocyte. Arterioscler Thromb Vasc. Biol. 2006;26:2260–2266. doi: 10.1161/01.ATV.0000240290.70852.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key NS, Slungaard A, Dandelet L, Nelson SC, Moertel C, Styles LA, Kuypers FA, Bach RR. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood. 1998;91:4216–4223. [PubMed] [Google Scholar]
- Lai JP, Douglas SD, Ho WZ. Human lymphocytes express substance P and its receptor. J Neuroimmunol. 1998;86(1):80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Zhan GX, Yi Y, Collman RG, Douglas SD. Substance P antagonist (CP-96,345) inhibits HIV-1 replication in human mononuclear phagocytes. Proc Natl Acad Sci U S A. 2001;98(7):3970–3975. doi: 10.1073/pnas.071052298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JP, Ho WZ, Kilpatrick LE, Wang X, Tuluc F, Korchak HM, Douglas SD. Full-length and truncated neurokinin-1 receptor expression and function during monocyte/macrophage differentiation. Proc Natl Acad Sci U S A. 2006;103(20):7771–7776. doi: 10.1073/pnas.0602563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey DR, Novak JM, Polonis VR, Liu Y, Gartner S. Characterization of substance P binding to human monocytes/macrophages. Clin. Diagn. Lab. Immunol. 1994;1:330–335. doi: 10.1128/cdli.1.3.330-335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151. [PubMed] [Google Scholar]
- Payan DG, Brewster DR, Missirian-Bastian A, Goetzl EJ. Substance P recognition by a subset of human T lymphocytes. J. Clin. Invest. 1984;74:1532–1539. doi: 10.1172/JCI111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost N, Woulfe D, Tanaka T, Brass LF. Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred. Proc Natl Acad Sci U S A. 2002;99:9219–9224. doi: 10.1073/pnas.142053899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger T, Bresser P, Snoek M, van der Sluijs K, van den Berg A, Nijhuis M, Jansen HM, Lutter R. Exaggerated IL-8 and IL-6 responses to TNF-alpha by parainfluenza virus type 4-infected NCI-H292 cells. Am J Physiol Lung Cell Mol Physiol. 2004;287(5):L1048–L1055. doi: 10.1152/ajplung.00396.2003. [DOI] [PubMed] [Google Scholar]
- Stanisz AM, Scicchitano R, Dazin P, Bienenstock J, Payan DG. Distribution of substance P receptors on murine spleen and Peyer’s patch T and B cells. J. Immunol. 1987;139:749–754. [PubMed] [Google Scholar]
- Shanahan F, Denburg JA, Fox J, Bienenstock J, Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J. Immunol. 1985;135:1331–1337. [PubMed] [Google Scholar]
- Satake H, Kawada T. Overview of the primary structure, tissue distribution, and functions of tachykinins and their receptors. Curr. Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- Tuluc F, Lai JP, Kilpatrick LE, Evans DL, Douglas SD. Neurokinin 1 receptor isoforms and the control of innate immunity. Trends in Immunology. 2009;30:271–276. doi: 10.1016/j.it.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Helps SC, Thornton E, Vink R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Research. 2011;1393:84–90. doi: 10.1016/j.brainres.2011.03.066. [DOI] [PubMed] [Google Scholar]
- van den Berg YW, Versteeg HH. Alternatively spliced tissue factor. A crippled protein in coagulation or a key player in non-haemostatic processes? Hamostaseologie. 2010;30(3):144–149. [PubMed] [Google Scholar]
- Wozniak A, McLennan G, Betts WH, Murphy GA, Scicchitano R. Activation of human neutrophils by substance P: effect on fMLPstimulated oxidative and arachidonic acid metabolism and on antibodydependent cell-mediated cytotoxicity. Immunology. 1989;68:359–364. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Berger A, Milne CD, Paige CJ. Tachykinins in the immune system. Curr. Drug Targets. 2006;7:1011–1020. doi: 10.2174/138945006778019363. [DOI] [PubMed] [Google Scholar]
- Zhu L, Bergmeier W, Wu J, Jiang H, Stalker TJ, Cieslak M, Fan R, Boumsell L, Kumanogoh A, Kikutani H, Tamagnone L, Wagner DD, Milla ME, Brass LF. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104:1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]