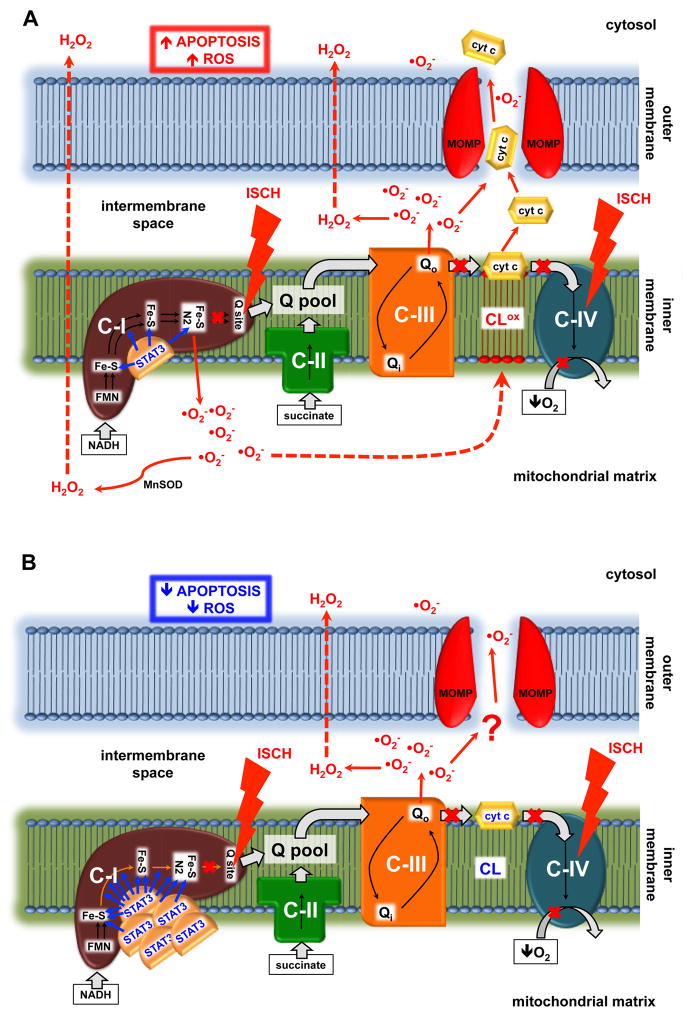
Figure 2. Postulated mechanism of the protective role of STAT3 in heart mitochondria during ischemia.
(A) In wild type mitochondria ischemia increases superoxide production from complex I that is directed toward the matrix. This results in cardiolipin oxidation and cytochrome c delocalization from the inner membrane. Further damage to the mitochondria leads to outer membrane permeabilization, which allows the release of cytochrome c from mitochondria and the subsequent induction of apoptosis. (B) Over expression of MLS-STAT3E in the mitochondria partially blocks electron flow through iron-sulfur clusters within complex I, resulting in blockade of superoxide generation from complex I during ischemia. This in turn decreases cardiolipin oxidation and preserves cytochrome c retention in the inner membrane. Less ROS and the lack of cytochrome c translocation into the cytosol attenuates apoptosis and increases cell viability during oxidative stress. STAT3, signal transducer and activator of transcription 3; C-I, II, III and IV, respiratory complex I, II, III and IV; cyt c, cytochrome c; Q, ubiquinone; FMN, flavin mononucleotide; Fe-S, iron-sulfur cluster; NADH, nicotinamide adenine dinucleotide; ISCH, ischemia; ROS, reactive oxygen species; MnSOD, mitochondrial manganese superoxide dismutase; CL, cardiolipin; CLox, oxidized cardiolipin; MOMP, mitochondrial outer membrane permeabilization.