Fig. 1.
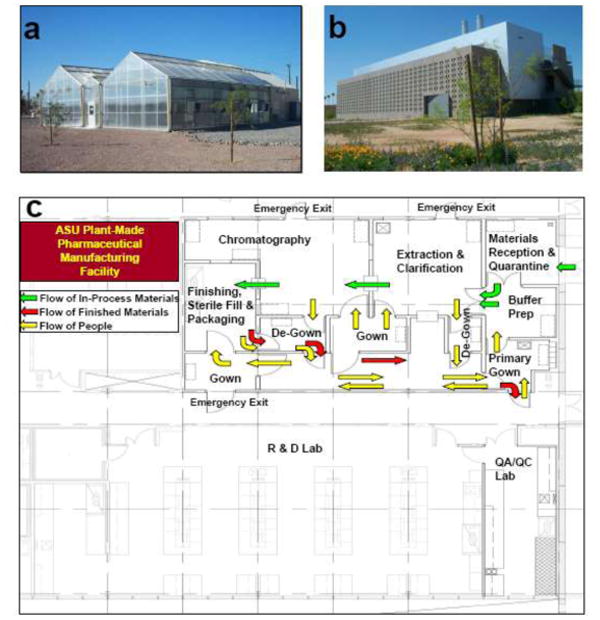
Facilities for cGMP production of NVCP VLP vaccine and other plant-made pharmaceuticals. (a) The 3600 ft2 BSL-2 greenhouse facility for plant biomass generation and NVCP expression. (b) The Plant Biopharmaceutical Center that houses the Central Bioprocessing Suite, the QA/QC laboratory, and the Process Development laboratory. (c) The floor plan of the Central Bioprocessing Suite. The arrows indicate the unidirectional flow of in-process materials (green), purified final product (red) and people (yellow).
