Abstract
Telomeres are regions of repetitive DNA at the end of eukaryotic chromosomes, which prevent chromosomal instability. Telomere shortening is linked to age-related disease including Alzheimer's disease (AD) and has been reported to be reduced in leukocytes of AD patients. The aim of the present study was to measure telomere length in monocytes of patients with AD or mild cognitive impairment (MCI) compared to healthy subjects. Our data show significant shorter telomere length in AD patients (6.6 ± 0.2 kb; p = 0.05) compared to controls (7.3 ± 0.2 kb). Telomere length of MCI patients did not differ compared to healthy subjects (7.0 ± 0.2 kb). We observe a strong correlation between telomere length and age (p = 0.01, r = − 0.38), but no association between telomere length and Mini-Mental State Examination score. In conclusion, the telomere length is age-dependent in monocytes and decreased in AD patients, which could mean that the AD pathology may contribute to telomere length shortening. The high variability of telomere lengths in individuals suggests that it will not be useful as a general biomarker for AD. However, it could become a biomarker in personalized long-term monitoring of an individuals’ health.
Keywords: Monocyte, Alzheimer, Blood, Diagnose, Telomere
Highlights
► Alzheimer‘s disease is a severe neurodegenerative disorder of the brain. ► Laboratory diagnosis of AD is difficult and not well established. ► Monocytes play a role in inflammation and may reflect AD pathology. ► Telomer length is reduced in monocytes of AD patients. ► Telomer length could be a putative (personalized) biomarker for diagnosis.
1. Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder which is characterised by cognitive impairment, memory loss and characteristic pathological changes in the brain, like senile plaques and neurofibrillary tangles (Burns et al., 2002). To complement diagnosis an intense search is underway to identify disease-specific biomarkers in the cerebrospinal fluid (CSF), blood plasma, and blood cells. To date, three biomarkers have been established in CFS: beta-amyloid1–42 (Aβ), total tau, and phospho-tau-181 (Humpel, 2011). So far no specific blood biomarkers have been established, despite an intense research on proteins and genes of blood cells (Humpel, 2011).
Telomeres are short and highly conserved hexanucleotide repeats (TTAGGG) found at the end of eukaryotic chromosomes, which prevent end-to-end fusions and other structural and functional cell abnormalities. During aging 50–150 bp of telomeric DNA is lost with each proliferation cycle (Allsopp et al., 1992). Shorter telomere length of leukocytes has been linked to age-related diabetes, cardiovascular and heart disease and also to an elevated risk of neurodegenerative disease including dementia (Honig et al., 2006; Panossian et al., 2003; Tentolouris et al., 2007; von Zglinicki et al., 2000). In particular, telomere shortening in white blood cells and altered immune function as a possible result has been linked to AD (Honig et al., 2006; Panossian et al., 2003; Thomas et al., 2008). Immune cells, like monocytes are further associated with Aβ depositions and are capable of phagocytosing Aβ (Fiala et al., 2007).
The objective of this study was to investigate, if telomere length in monocytes is altered in patients with AD or MCI compared to healthy subjects. If so, these results will provide a basis to further investigate monocytic involvement in the pathology of AD, which could help to use telomere length to distinguish between healthy subjects and MCI, or AD patients.
2. Methods
2.1. Selection of patients
Healthy subjects and patients suffering from AD or MCI were recruited from the Department of Psychiatry in Innsbruck or Klagenfurt, Austria. All groups were assessed by the same diagnostic procedure. Psychiatrists clinically examined all subjects, performed a standardized neurological examination, neuropsychological tests (Mini-Mental State Examination, MMSE), reviewed medical records, and conferred with referring physicians for all patients. MCI was diagnosed according to the Petersen criteria (Petersen et al., 2001). Probable AD was diagnosed according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria (McKhann et al., 1984). The geriatric depression scale (GDS) was applied to all participants. Magnetic resonance imaging was performed for all participants. Subjects were excluded when they suffered from another mental disease, any kind of metabolic decompensation or had any signs of inflammation. The study was approved by the ethical committee of Innsbruck Medical University.
2.2. Monocyte collection
Monocytes were isolated as described recently in detail (Hochstrasser et al., 2010). Briefly, EDTA blood (10 ml) was collected during normal routine clinical assessments and processed within 3 h. Plasma and peripheral mononuclear cells (PBMCs) were separated from whole blood on a continuous Biocoll gradient (1.077 g/ml, Biochrom, Germany) after centrifugation (400 ×g, 30 min, room temperature). Two-thirds of the upper plasma phase and the interphase with the PBMCs, which is visible as a white stratum between plasma phase and Biocoll, were carefully removed. Plasma was directly frozen at − 80 °C until use. PBMCs were washed in 50 ml phosphate-buffered saline (PBS), centrifuged (250 ×g, 6 min) and the pellet was dissolved in PBS with 1% bovine serum albumine (BSA). Monoyctes were isolated by negative magnetic isolation as described by the manufacturer (Miltenyi Biotech, Germany). Briefly, PBMCs were incubated with a cocktail of different biotinylated antibodies (CD3, CD7, CD16, CD19, CD56, CD123, CD235a) for 10 min on ice. Then anti-biotin magnetic beads were added, incubated for further 15 min on ice, washed and the cells applied onto MACS MS columns (Miltenyi Biotech, Germany) on a strong magnet. The non-labelled monocytes were eluted and collected. Finally, cells were frozen at − 80 °C until use.
2.3. Telomere length assay
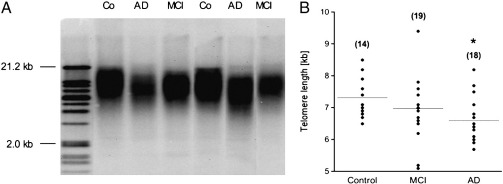
Telomere length analysis was performed by the TeloTAGGG telomere length assay kit (Roche, Austria) as described by the manufacturer. Briefly, genomic DNA was extracted from monocytes using the QIAamp DNA mini kit (Qiagen, Austria) according to the manufacturer's instructions. DNA was digested by the restriction endonucleases HinfI and RsaI for 2 h at 37 °C. Following DNA digestion, the DNA fragments were separated by gel electrophoreses (0.8% agarose gel, 50 V, 3 h). Then, DNA was transferred to a positively charged nylon membrane (Roche, Austria) by capillary Southern blotting with 20 × saline-sodium citrate (SSC) buffer (overnight, room temperature). DNA was fixed to the membrane by UV light for 5 min, washed with 2 × SSC buffer and air dried. DNA fragments were hybridized with telomeric specific digoxygenin (DIG)-labeled hybridization probe (3 h, 42 °C), incubated with anti-DIG-alkaline phospahatase for 30 min at room temperature and detected with CDP-Star chemiluminescent substrate. The signals were analyzed using a CCD imager. Telomere length is given as the average terminal restriction fragment (TRF) length and the signal intensity is plotted in function of migrating distance for each sample. Mean TRF length was quantified by integrating the signal intensity of the TRF bands on the blot as a function of its mean molecular weight, which is determined based on the molecular weight standard (Fig. 1A).
Fig. 1.
Telomere length analysis. (A) A representative Southern Blot shows telomere lengths (TRF) from monocytes of control subjects (Co), Alzheimer's disease (AD) and mild cognitive impaired (MCI) patients. A size marker on the left gives the size of DNA fragments in kilo bases (kb) (B) Scatter plot shows the telomere length of controls, MCI, and AD patients. Mean telomere length from AD patients is shorter (*p = 0.05) than from controls. Value in parenthesis gives the number of samples.
2.4. SearchLight Multiplex ELISA
SearchLight Multiplex ELISAs (Aushon Biosystems) for plasma pro-inflammatory cytokines tumor necrosis factor-alpha (TNFα), RANTES, interleukin-1 alpha (IL-1α), and monocyte chemotactic protein-3 (MCP3) were performed as described by us in detail (Marksteiner et al., 2011).
2.5. Statistical analysis
Sample size considerations for our study are based on the study of Panossian et al. (2003) who found a fairly large difference in monocyte telomere length between AD patients and healthy controls compared to the standard deviation (SD) in each of the two groups (mean difference = 0.91 kb, SD < 0.5 kb, giving rise to an effect size d > 1.8; d = mean difference/SD). Our sample size of 19 patients with AD, 18 with MCI and 14 healthy controls was chosen such that in a two sample t-test (AD vs controls, MCI vs. controls) effect sizes d > 1 can be detected with 80% power at a 5% level of significance. Considerations for ANCOVA are similar (detectable effect size d ≈ 1.1, 1.2, 1.3 for one, two and three covariates, respectively).
Statistical analysis was performed with analysis of variance (ANOVA) followed up by post-hoc pairwise comparisons of groups using Fisher's least significant difference (LSD) method. The ability of the telomere length to discriminate between diagnostic groups was tested by analysis of covariance (ANCOVA). ANCOVA was conducted in order to adjust for age, sex, and GDS. The correlation of telomere length to age or MMSE was assessed by ANCOVA, where p < 0.05 and was considered as statistically significant.
3. Results
Subjects’ characteristics are presented in Table 1. Healthy controls did not show a difference in sex, age, and GDS compared to MCI and AD patients (Table 1). Controls had an MMSE score of 28.4 ± 0.4, which was significantly different from AD but not from MCI patients (Table 1). Pro-inflammatory markers were measured by SearchLight ELISA in plasma, but no significant difference was observed between controls, MCI and AD patients (Table 2).
Table 1.
Patients characteristics for telomere length.
| n | m/f | Age | MMSE | GDS | |
|---|---|---|---|---|---|
| Control | 14 | 6/7 | 71.9 ± 1.9 | 28.4 ± 0.4 | 6.1 ± 1.2 |
| MCI | 19 | 6/13 ns | 71.2 ± 1.6 ns | 27.4 ± 0.4 ns | 9.5 ± 1.4 ns |
| AD | 18 | 4/14 ns | 74.7 ± 1.7 ns | 19.6 ± 1.1 *** | 10.1 ± 1.4 ns |
Monocytes were isolated from healthy controls, mild cognitive impaired (MCI), and Alzheimer's disease (AD) patients. Values are given as mean ± SEM. n gives the number of samples and the number of respective male/female patients (m/f) is given. The table gives the age of the patients in years, the Minimental State Examination (MMSE) and the geriatric depression scale (GDS). Statistical analysis was performed by one-way ANOVA with a Fisher LSD post-hoc test. *** p < 0.001; ns not significant.
Table 2.
Inflammatory marker in plasma of controls, MCI, and AD patients.
| TNFα [pg/ml] | RANTES [ng/ml] | IL-1α [pg/ml] | MCP3 [pg/ml] | |
|---|---|---|---|---|
| Control | 8.2 ± 1.1 (7)– | 10.5 ± 3.2 (14)– | 3.1 ± 0.9 (7)– | 3.4 ± 1.5 (7)– |
| MCI | 11.4 ± 2.4 (9) ns | 10.4 ± 2.8 (18) ns | 2.3 ± 0.4 (9) ns | 1.5 ± 0.1 (9) ns |
| AD | 10.7 ± 1.8 (12) ns | 12.8 ± 4.0 (18) ns | 2.5 ± 0.3 (12) ns | 2.2 ± 0.3 (12) ns |
Plasma was taken from healthy controls, mild cognitive impaired (MCI), and Alzheimer's disease (AD) patients. Plasma was analyzed for detection of inflammatory markers (tumor necrosis factor-alpha, TNFα; RANTES; interleukin-1 alpha, IL-1α; monocyte chemotactic protein-3, MCP-3) by SearchLight Multiplex ELISA. Values are given as mean ± SEM pg/ml or ng/ml. The number of analyzed samples is given in parenthesis. Statistical analysis was performed by one-way ANOVA. ns, not significant.
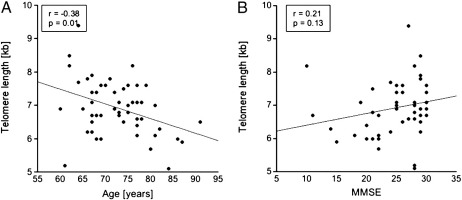
The telomere length in monocytes was determined by Southern blotting and was found to be between 6 and 7 kilobases (Fig. 1A). In order to measure size differences, the blots were scanned and the DNA size was blotted against the running distance related to 21.2 kb, which yielded in a formula with a very high regression coefficient (R2 = 0.99). The size of the monocyte telomere length was calculated accordingly, and the monocyte telomere length was found to be 7.3 ± 0.2 kb in healthy subjects (n = 14; Fig. 1B). For MCI patients, the telomere length was 7.0 ± 0.2 kb (n = 19) and AD patients had a mean monocyte telomere length of 6.6 ± 0.2 kb (n = 18) (Fig. 1B). When telomere length was adjusted for age, the analysis of covariance revealed an all over trend towards shorter telomeres when controls were compared to MCI and AD patients (p = 0.08). Telomere length of MCI patients did not significantly differ from telomere lengths of healthy controls (p = 0.15; Bonferroni-corrected: p = 0.30). AD patients showed a significance towards shorter telomeres (p = 0.03; Bonferroni-corrected: p = 0.05) compared to healthy controls. A significant negative correlation was seen between telomere length and age in monocytes of all groups (Fig. 2A; r = − 0.38, p = 0.01), while there was no correlation between telomere length and MMSE score (Fig. 2B; r = 0.21, p = 0.13). There was no significant association between the telomere length and GDS (r = − 0.08; p = 0.6), or sex (p = 0.34) adjusted for age.
Fig. 2.
Correlation of telomere length to age or to cognitive function (MMSE). The telomere length of monocytes is plotted against (A) age in years (r = − 0.376, p = 0.007, analysis of covariance), or (B) MMSE score (r = 0.213, p = 0.134, analysis of covariance). r = correlation coefficient.
4. Discussion
Our findings indicate a significant reduction in telomere length in monocytes of AD patients compared to healthy subjects.
Short telomeres have been found as a marker for biological aging and several studies have shown an association between short telomeres and age-related disease such as dementia (Honig et al., 2006; Panossian et al., 2003; Thomas et al., 2008; von Zglinicki et al., 2000). Telomere shortening was first experimentally demonstrated in fibroblasts and has been shown to be associated with aging (Baird, 2006). Here, we report that monocytes of AD patients show shorter telomeres, which are marginally significant when adjusted for age. MCI patients displayed no difference in monocyte telomere length compared to control subjects. A correlation between peripheral blood leukocytes and cerebellum telomere length, a correlation between leukocyte telomere length and cognitive performance, and increased telomerase activity and decreased proliferation activity in lymphocytes has been demonstrated in AD patients (Lukens et al., 2009; Valdes et al., 2010). We did not observe a correlation between cognitive performance (MMSE) and monocyte telomere length, which is in agreement with others (Valdes et al., 2010). However, they show lower MMSE scores in T cell telomere length of AD patients compared to controls. In order to measure the pro-inflammatory status, we analyzed four well established markers in plasma but did not find a change in inflammation in AD and MCI patients.
We observed a significant negative correlation between telomere length and age, which points to an age-related telomere shortening, rather than to a disease-specific event. In consistence, cerebellum telomere length correlates inversely to age in AD patients (Lukens et al., 2009). It is suggested, that telomere length is not the major determinant of AD, since individuals would develop AD as soon as telomeres shorten to a certain size (Lukens et al., 2009). However, it is still not completely clear if and how telomere shortening contributes to AD. It cannot be determined whether these changes in telomere length are the result in response to AD brain pathology, such as tissue damage, or if reduced telomere length somehow contributes to AD. We found a correlation between age and telomere length and a reduced telomere length in AD patients suggesting that age is the major contributor to telomere shortening. However, AD and other neurodegenerative diseases, such as vascular dementia and Parkinson's disease are associated with telomere length shortening (von Zglinicki et al., 2000; Guan et al., 2008). It seems likely that long-term chronic inflammation and/or oxidative stress contributes to telomere shortening in monocytes (von Zglinicki et al., 2000; Guan et al., 2008). In addition telomere length highly correlates to aging, and telomeres might be more vulnerable in old age and then contribute to AD development. Further research regarding these findings in large, longitudinal prospective studies is needed. Interestingly, microglia also display shorter telomeres in the AD brain, suggesting that these cells undergo early replicative senescence, which could be induced by the severe amyloid plaque overload in AD (Flanary et al., 2007). Monocytes migrate through the blood-brain barrier in AD and convert into microglia cells in the brain and microglial activation has been reported in association with amyloid-plaques in the AD brain (Floris et al., 2002; Hickman and El Khoury, 2010; Malm et al., 2005). Additionally, increased expression of chemokine receptors and cytokines in peripheral blood mononuclear cell of AD patients has been shown (Reale et al., 2008).
In conclusion, we observed a strong correlation between age and telomere length and we found a significant shorter telomere length in monocytes of AD patients. This may suggest that the AD pathology further enhances telomere shortening. Regardless of how telomere length is associated to AD, we assume that telomere length of monocytes could serve as a biomarker for AD in a personalized long-term monitoring of an individuals’ health. However, there are limitations for a general biomarker, because telomere length is strongly correlated to age and the variability of the telomere length between individuals is very high.
Acknowledgments
This study was supported by Austrian Science Funds (L429-B05). TH was supported by the European Community (Moodinflame, Early diagnosis, treatment and prevention of mood disorders targeting the activated inflammatory response system. FP7-Health-2007 Project no: 222963). We thank Dr. Georg Kemmler for help with statistical analysis and Ursula Kirzenberger-Winkler for excellent technical assistance. All authors reported NO biomedical financial interests or potential conflict of interest.
Section Editor: Andrzej Bartke
References
- Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C.W., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. U. S. A. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird D.M. Telomeres. Exp. Gerontol. 2006;41:1223–1227. doi: 10.1016/j.exger.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Burns A., Byrne E.J., Maurer K. Alzheimer's disease. Lancet. 2002;360:163–165. doi: 10.1016/S0140-6736(02)09420-5. [DOI] [PubMed] [Google Scholar]
- Fiala M., Cribbs D.H., Rosenthal M., Bernard G. Phagocytosis of amyloid-beta and inflammation: two faces of innate immunity in Alzheimer's disease. J. Alzheimers Dis. 2007;11:457–463. doi: 10.3233/jad-2007-11406. [DOI] [PubMed] [Google Scholar]
- Flanary B.E., Sammons N.W., Nguyen C., Walker D., Streit W.J. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Floris S., Ruuls S.R., Wierinckx A., van der Pol S.M., Döpp E., van der Meide P.H., Dijkstra C.D., De Vries H.E. Interferon-beta directly influences monocyte infiltration into the central nervous system. J. Neuroimmunol. 2002;127:69–79. doi: 10.1016/s0165-5728(02)00098-x. [DOI] [PubMed] [Google Scholar]
- Guan J.Z., Maeda T., Sugano M., Oyama J., Higuchi Y., Suzuki T., Makino N. A percentage analysis of the telomere length in Parkinson's disease patients. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:467–473. doi: 10.1093/gerona/63.5.467. [DOI] [PubMed] [Google Scholar]
- Hickman S.E., El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease. CNS Neurol. Disord. Drug Targets. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser T., Weiss E., Marksteiner J., Humpel C. Soluble cell adhesion molecules in monocytes of Alzheimer's disease and mild cognitive impairment. Exp. Gerontol. 2010;45:70–74. doi: 10.1016/j.exger.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig L.S., Schupf N., Lee J.H., Tang M.X., Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann. Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens J.N., Van Deerlin V., Clark C.M., Xie S.X., Johnson F.B. Comparisons of telomere lengths in peripheral blood and cerebellum in Alzheimer's disease. Alzheimers Dement. 2009;5:463–469. doi: 10.1016/j.jalz.2009.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm T.M., Koistinaho M., Pärepalo M., Vatanen T., Ooka A., Karlsson S., Koistinaho J. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 2005;18:134–142. doi: 10.1016/j.nbd.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Marksteiner J., Kemmler G., Weiss E.M., Knaus G., Ullrich C., Mechtcheriakov S., Oberbauer H., Auffinger S., Hinterhölzl J., Hinterhuber H., Humpel C. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2011;32:539–540. doi: 10.1016/j.neurobiolaging.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Panossian L.A., Porter V.R., Valenzuela H.F., Zhu X., Reback E., Masterman D., Cummings J.L., Effros R.B. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiol. Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V., Ritchie K., Rossor M., Thal L., Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Reale M., Iarlori C., Feliciani C., Gambi D. Peripheral chemokine receptors, their ligands, cytokines and Alzheimer's disease. J. Alzheimers Dis. 2008;14:147–159. doi: 10.3233/jad-2008-14203. [DOI] [PubMed] [Google Scholar]
- Tentolouris N., Nzietchueng R., Cattan V., Poitevin G., Lacolley P., Papazafiropoulou A., Perrea D., Katsilambros N., Benetos A. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;11:2909–29015. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- Thomas P., O' Callaghan N.J., Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech. Ageing Dev. 2008;129:183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Valdes A.M., Deary I.J., Gardner J., Kimura M., Lu X., Spector T.D., Aviv A., Cherkas L.F. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol. Aging. 2010;31:986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T., Serra V., Lorenz M., Saretzki G., Lenzen-Grossimlighaus R., Gessner R., Risch A., Steinhagen-Thiessen E. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab. Invest. 2000;80:1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]