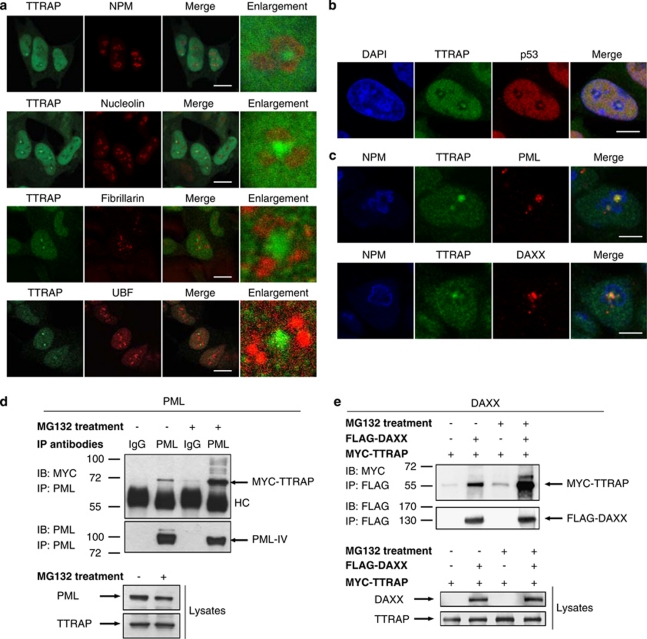
Figure 2.
TTRAP associates with PML-NBs in nucleolar cavities and interacts with PML and PML-NBs component DAXX. (a) Nucleolar localization of TTRAP in MG132-treated SH-SY5Y cells. SH-SY5Y cells were treated for 16 h with 5 μM MG132. Double immunofluorescence was performed with anti-TTRAP (green) and nucleolar markers (red) NPM, NCL, Fibrillarin or UBF, as indicated. Colocalization is shown in merge. TTRAP location relative to nucleolar marker is highlighted in enlarged images. Bars, 10 μm. (b) TTRAP colocalizes with p53 in nucleolar cavities. SH-SY5Y cells were treated as in (a). Double immunofluorescence was performed with anti-TTRAP (green) and anti-p53 (red) antibodies. Colocalization between TTRAP and p53 is shown in merge. Bars, 5 μm. (c) TTRAP colocalizes with components of PML-NBs in the nucleolus. Cells as in (a). Triple immunofluorescence was performed with anti-NPM (blue), anti-TTRAP (green) and anti-PML or anti-DAXX (red) antibodies. Colocalization between the three proteins is shown in merge. Bars, 5 μm. (d) HEK-293T cells were transfected with MYC-TTRAP and PML IV. After transfection, cells were left untreated or incubated with 5 μM MG132 for 16 h, as indicated. Cell lysates were immunoprecipitated (IP) with anti-PML or control mouse IgG and bound proteins were revealed by immunoblot (IB) with anti-MYC and anti-PML antibodies. An aliquote of each lysate was tested for the expression of PML and TTRAP proteins, as indicated. Molecular weight markers are indicated on the left (kDa). HC, heavy chain. (e) HEK-293-T cells were transfected with FLAG-DAXX and MYC-TTRAP. Cells were treated as in (d). Cell lysates were immunoprecipitated with anti-FLAG agarose beads and bound proteins were revealed with anti-FLAG and anti-MYC antibodies