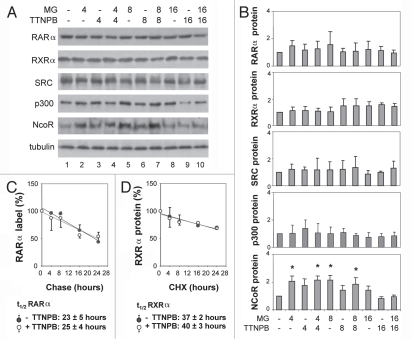
Figure 2.
Short term of ligand induction does not affect the stability of RARα. (A) Equal amounts of whole cell extracts (50 µg) were used for western blot analysis of the endogenous RARα, RXRα, SRC-1, p300 and NcoR proteins following treatments with TTNPB (1 µM) for 4, 8 and 16 h in the presence or absence of MG132 (MG, 5 µM). The blots were then stripped and re-probed with a γ-tubulin antibody for internal control. (B) Quantitative analysis of the blots as in (A) is expressed as fold variations compared with the untreated controls after being normalized to the loading controls. Error bars represent standard deviations of at least three independent experiments (*p < 0.05). (C) After pulse labeling, the cells were chased in the presence or absence of ligand for 4–24 h. The endogenous RARα proteins were immunopurified, separated by SDS-PA GE and quantified by PhosphorImager. The apparent half-life of endogenous RARα is 23 ± 5 hours in the absence of ligand and 25 ± 4 h in the presence of ligand (n = 3). (D) The cells were treated with cycloheximide (10 µg/ml) in the presence or absence of ligand for 4–24 h and harvested for western analysis of the endogenous RXRα. The apparent half-life of endogenous RXRα is 37 ± 2 h in the absence of ligand and 40 ± 3 h in the presence of ligand (n = 3).