Abstract
Chronic pain following spinal cord injury (SCI) is a highly prevalent clinical condition that is difficult to treat. Using both von Frey filaments and radiant infrared heat to assess mechanical allodynia and thermal hyperalgesia, respectively, we have demonstrated that a one-time injection of fibronectin (50 μg/mL) into the spinal dorsal column (1 μL/min each injection for a total of 5 μL) immediately after SCI inhibits the development of mechanical allodynia (but not thermal hyperalgesia) over an 8-month observation period following spinal cord dorsal column crush (DCC). DCC will only induce mechanical Allodynia, but not thermal hyperalgesia or overt motor deficits. By applying various fibronectin fragments as well as competitive inhibitors, these effects were shown to be dependent on the connecting segment-1 (CS-1) motif of fibronectin. Furthermore, we found that acute fibronectin treatment diminished inflammation and blood–spinal cord barrier permeability, which in turn leads to enhanced fiber sparing and sprouting. In particular, the reduction of serotonin (5-HT) in the superficial dorsal horn, an important descending brainstem system in the modulation of pain, was blocked with fibronectin treatment. We conclude that treatment of SCI with fibronectin preserves sensory regulation and prevents the development of chronic allodynia, providing a potential therapeutic intervention to treat chronic pain following SCI.
Key words: fibronectin; inflammation, mechanical allodynia; serotonin; thermal hyperalgesia
Introduction
Chronic pain, which disturbs behavioral function and reduces the quality of life, has emerged as a major challenge in the treatment of spinal cord injury (SCI). Neuropathic pain such as allodynia (non-noxious stimuli become noxious), and hyperalgesia (noxious stimuli become more noxious) serve no protective function (Vickers and Cousins, 2000), and no cure is currently available for chronic pain (Baastrup and Finnerup, 2008). Furthermore, addiction to pain medication due to continuous administration or abuse can occur (Anderson, 2004, Jensen et al., 2009). In order to improve the prognosis and quality of life of SCI patients, novel strategies to inhibit chronic SCI pain are needed.
The mechanisms underlying the development of SCI pain are complex (Finnerup, 2008; Fouad et al., 2011; Sandkuhler, 2009). Inflammation, which accompanies SCI, is a potential source of cytokines, proteases, and signaling molecules, that lead to secondary damage (Alexander and Popovich 2009; Fitch et al., 1999). Indeed, the complicated and interactive central injury cascade can lead to “dying back” neuropathy (Busch et al., 2009), as well as axotomy (Bareyre et al., 2004; Fitch and Silver, 2008) beyond that produced by the initial injury (Jefferson et al., 2011), causing an exaggerated interruption of a variety of descending systems, including those that modulate pain such as the serotonin (5-HT) system (Hains et al., 2003).
Integrins physically connect cells to the extracellular matrix and stimulate a variety of signaling cascades that regulate cell migration and neurite extension or sprouting (Milner and Campbell, 2002). The roles of integrins in mediating lymphocyte, neutrophil, and monocyte extravasation during central nervous system (CNS) inflammation have been extensively studied (Luo et al., 2007). After injury, both integrins α4β1 and α5β1, receptors for fibronectin, are associated with maintenance of the vasculature and are important for the infiltration of inflammatory cells into the injured spinal cord (Milner and Campbell, 2002). Furthermore, integrin α4β1 and α5β1 have been identified (Tomaselli et al., 1993) in primary afferent neurons that mediate pain via the spinothalamic system (Andrew et al., 1992).
There are several models of chronic pain studies including: (1) an ischemia model (Hao et al., 1992); (2) unilateral quisqualate intraspinal injection (Wolfe et al., 2007); (3) spinal contusion (Christensen et al., 1996; Hulsebosch et al., 2009; Mills et al., 2001); (4) anterolateral tract lesions (Vierck and Light, 1999); (5) spinal hemisection (Christensen and Hulsebosch, 1997); and (6) spinal clip compression (Fleming et al., 2008). All models elicit varying levels of allodynia within a relatively short observation period. In the current report, a dorsal column crush (DCC) injury was used as a model of chronic SCI-induced pain. Indeed, while the dorsal column system is well known for its transmission of light touch sensation, the existence of long ascending unmyelinated primary afferent fibers within the dorsal columns (Patterson et al., 1992), which play critical roles in visceral nociception (Willis and Westlund, 2001) is a lesser known and clinically relevant component of this ascending sensory tract.
We demonstrate that fibronectin, when intraspinally injected immediately after DCC, significantly inhibited the inflammatory response, and subsequently remarkably reduced the development of mechanical allodynia (but not thermal hyperalgesia) in the hindpaws of rats over an 8-month observation period. Although the molecular mechanisms of this therapeutic effect are not completely clear, we found that at the cellular level of analysis, fibronectin treatment decreased blood–spinal cord barrier (BSCB) breakdown and inflammatory cell invasion. Also, fibronectin treatment fully blocked the DCC-induced reduction of 5-HT that typically occurs in the vicinity of and distal to the lesion. These results demonstrate that a one-time fibronectin treatment could engage integrin-mediated signaling to influence the descending 5-HT modulatory system and inflammation, which could conceivably lead to an unprecedented amount of inhibition of chronic SCI pain.
Methods
Animal subjects
Pathogen-free adult male Sprague-Dawley rats (200–250 g; Harlan Laboratories, Madison, WI) were used in all experiments. The rats were housed in standard laboratory cages under 12-h/12-h light/dark cycle conditions with standard rodent chow and water available ad libitum. The experiments were performed during the light cycle. The animals were divided into groups based on the type of treatment received. Additional animals without lesion or treatment served as controls for individual experiments. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic.
DCC lesion and subsequent treatment
The rats were anesthetized with isoflurane gas (2% in oxygen) for all surgical procedures. The dorsal aspect of the C8 spinal cord segment was exposed by performing a T1 laminectomy. Two holes were made bilaterally 0.75 mm from the midline with a 30-gauge needle. A DCC lesion was then made by inserting a Dumont #3 forceps into the dorsal spinal cord at C8 to a depth of 1.0 mm and squeezing the forceps, holding pressure for 10 sec. This crushing procedure was repeated two additional times. Immediately after the lesion, 5 μL (50 μg/mL) of fibronectin (n=8, full-length, F0635; Sigma-Aldrich, St. Louis, MO), connecting segment-1 (CS-1, n=8; Bachem), a 50-kDa fragment (n=5; a gift from Dr. Sue Craig, The University of Manchester, Manchester, U.K.), a 120-kDa fragment (n=5, F1904; Millipore, Billerica, MA), vehicle (n=10), or N-1765 [n=3; 4-(((2-methylphenyl)aminocarbonyl)-aminophenyl) acetyl-fibronectin CS-1 fragment; Bachem] were injected using a pulled glass micropipette attached to a Picospritzer II (General Valve, Fairfield, NJ). The injection was made into the lesion space as well as at 2 sites 1 mm both rostral and caudal to the lesion into the spinal cord at a depth of 1 mm (1.0 μL at each site), at a rate of 1 mL/min. The pipettes were left in place for an additional 1 min after the injection, and then were slowly withdrawn. Gel film was placed over the lesion to cover the holes in the dura. The muscle layers were closed with 4-0 nylon sutures, and the skin was closed with surgical staples. Upon completion of the surgery, the animals received buprenorphine (0.05 mg/kg) subcutaneously for 2 days to reduce pain. The animals were kept warm post-operatively with a heating pad during recovery from anesthesia and allowed access to food and water ad libitum. Additional rats received two doses (IP injections 24 h apart) of p-CPA (300 mg/kg each dose, a 5-HT synthesis inhibitor; Sigma-Aldrich; Hadley et al., 1999) or methysergide maleate (4 mg/kg each dose, a 5-HT1/5-HT2-receptor blocker; Sigma-Aldrich; Ochi et al., 2002), or an equal volume of a saline vehicle injection 2 weeks and 5 weeks after DCC with or without treatment. Another group of rats was injected at 5 weeks after DCC with or without fibronectin treatment. All groups of rats receiving p-CPA, methysergide, or saline injections were subjected to behavioral testing for pain-associated allodynia and hyperalgesia before and after the injection, and then perfused for immunohistochemical analysis of 5-HT expression.
Mechanical allodynia
Sensitivity to non-noxious mechanical stimuli was tested by assessing the hindpaw withdrawal threshold to a series of calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) used on the plantar surface of the hindpaw (Sun et al., 2001). The animals were acclimated for a period of 15 min in suspended 6-mm wire mesh cages. Baseline testing was performed 2 and 3 days prior to surgery to ensure that the animals showed normal responses. Groups of rats were assessed for mechanical allodynia before and after DCC every 7 days for 3–8 months. The plantar surface of the hindpaw of rats was stimulated with von Frey filaments perpendicularly. The filaments were applied to the hindpaw surface until the filament bent. Filaments with sequentially increasing or decreasing force were used to determine the withdrawal threshold; if a rat did not withdraw its hindpaw after two applications of a given filament, the next stiffer filament was tested. When the rat withdrew its hindpaw, the measurement was verified by ensuring that there was an absence of a response at the next lower filament. The procedure was repeated three times at 5-min intervals. A maximal cut-off of 100 g was employed to prevent potential tissue damage. The average gram force of the filament that caused a hindpaw withdrawal was recorded and expressed as the mean withdrawal threshold. This decreased hindpaw withdrawal threshold was the indicator for mechanical allodynia.
Thermal hyperalgesia
To assess paw withdrawal latency to a thermal nociceptive stimulus, the rats were first acclimated within a acrylic glass enclosure on a clear glass plate (Stoelting Co.). A radiant infrared heat source (i.e., high-intensity projector lamp) was activated with a timer and positioned directly beneath the plantar surface of the hindpaw (Sun et al., 2001). When the animal withdrew its hindpaw, the heat source was automatically switched off and the timer stopped. This allowed recording of the withdrawal latency. The latency to withdraw the hindpaw from the radiant heat source was determined both before and after treatment. A maximal cut-off of 40 sec was employed to prevent potential tissue damage. The thermal hyperalgesia was tested immediately after the tests for mechanical allodynia on the same day. We evaluated mechanical allodynia before and after DCC every 7 days for up to 8 months.
Determination of BSCB permeability
BSCB permeability was assessed 2 days or 9 days after DCC using previously described techniques (Fabis et al., 2008). Sodium fluorescein (NaF, 376-Da, 1 mL of 100 mg/mL in phosphate-buffered saline (PBS; Sigma-Aldrich) was injected IP and allowed to circulate in the rats (n=4 each group) for 30 min prior to transcardial perfusion with PBS and tissue collection. Spinal cord tissues were homogenized in PBS, centrifuged, and the supernatants clarified by using 7.5% trichloroacetic acid. NaOH was added to a final concentration of 1 M, and fluorescence at 485-nm excitation and 530-nm emission was determined by a spectraMax M2e microplate/cuvette reader (Molecular Devices, Downingtown, PA). We prepared 10 dilutions of an NaF standard, ranging from 500 mg to 30.5 ng, which is representative of the NaF solution to be tested. A linear equation obtained from the standard curve describing the absorbance-concentration relationship was used to calculate the NaF content in spinal cord (presented as milligrams NaF per milligram of tissue protein).
Tissue sectioning and immunohistochemistry
At the designated time points, groups of rats were transcardially perfused and spinal cords were processed for spatial and temporal changes in 5-HT staining intensity. The areas collected for sectioning included the lesion epicenter (0.7 cm long), and 0.7-cm-long sections taken both rostral and caudal to the lesion, unless stated otherwise. Transverse cryostat sections (30-μm thick) were mounted in serial order. There were six sections randomly selected from each area. The sections were blocked in 3% normal goat serum, 0.05 Triton X-100, and 1% bovine serum albumin (BSA) in PBS for 1 h. After blocking, the sections were incubated with anti-5-HT polyclonal antibody (DiaSorin, Stillwater, MN) overnight at room temperature. The sections were then washed, incubated with anti-rabbit secondary antisera conjugated with Alexa-Fluor 594 (Molecular Probes, Seattle, WA) in PBS/BSA for 1 h, and then cover-slipped with Vectashield (Vector Laboratories Inc., Burlingame, CA). Photographs were taken using a camera connected to a Leica DM5500 microscope.
Quantification of immunoreactive intensity and statistical analyses
Standardized areas for sampling in sections from each animal in each group were selected using Photoshop and Image J software. The mean number of pixels containing immunoreactive product in the sampled area was measured and multiplied by the average intensity. This value was subtracted from background immunolabeled intensity, as measured in a separate adjacent section. Graphs were plotted and statistics were assessed using Graphpad Prism 4.0 software (GraphPad, La Jolla, CA). Mean values for each animal were then normalized to obtain a percentage intensity value for each group of rats, and statistically analyzed using a paired t-test or a one-way analysis of variance (ANOVA) with Tukey's post-hoc test, with p≤0.05 considered significant. The intensities are shown as mean±standard deviation (SD) in units of the percentage of the mean intensities from normal rats, which were set at 100%. Significant differences between groups are indicated by asterisks (*p<0.05, **p<0.01, ***p<0.001 versus vehicle-treated rats), unless stated otherwise. Light intensity and threshold values were maintained at constant levels for all analyses. Analyses were conducted in a blinded fashion.
Results
Fibronectin blocked mechanical allodynia in the hindpaws of DCC rats
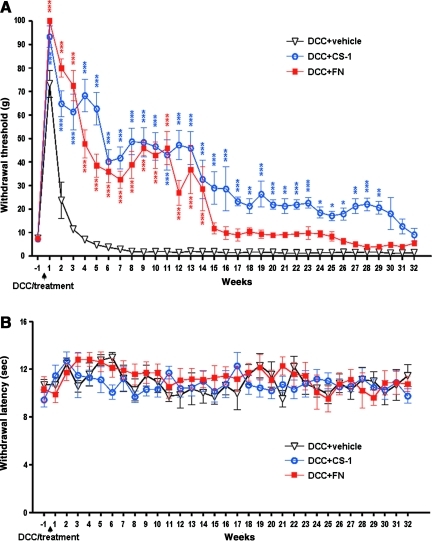
To examine whether fibronectin has potential therapeutic effects on reducing abnormal pain perception following SCI, a new rat DCC model was used. We evaluated mechanical allodynia before and after DCC every 7 days for up to 8 months. Rats with C8 DCC lost sensitivity 1 week post-DCC, and then slowly developed mechanical allodynia after 5 weeks post-DCC, which persisted throughout the experimental period. Hindpaw withdrawal thresholds in the range of 4.82±2.6 g (5 weeks post-DCC) became further reduced to 1.42±0.54 g (4 months post-DCC), and then stabilized at that low level (1.41±0.5 g, 8 months post-DCC; Fig. 1A). This value was significantly lower than the pre-DCC baseline (7.667±0.5 g), which is a strong indicator of the development of mechanical allodynia. Intraspinal injection of either full-length fibronectin or the 8-amino-acid connecting segment-1 (CS-1, the binding motif for integrin α4β1) resulted in desensitization and a complete blockade of mechanical allodynia in the hindpaws over the 25-week observation period post-DCC. Fibronectin treatment resulted in hindpaw withdrawal thresholds significantly increased to a range of 38.5±13.6 g (5 weeks post-DCC) to 9.88±7.5 g (4 months post-DCC), and only slightly decreased 26 weeks after DCC to 5.5±2.6 g (8 months post-DCC; Fig. 1A), values significantly improved compared to the vehicle-treated group (Fig. 1A). In particular, the hindpaw withdrawal thresholds were significantly increased with CS-1 treatment over the entire observation period, in the range of 62.63±19.4 g (5 weeks post-DCC) to 28.63±26.15 g (4 months post-DCC), and 9.13±7.7 g (8 months post-DCC; Fig. 1A), values significantly higher than both their pre-DCC baseline (7.33±1.2 g), and the vehicle-treated group. These data indicate that CS-1-treated rats are desensitized and never develop mechanical allodynia over an 8-month observation period.
FIG. 1.
Fibronectin treatment blocks mechanical allodynia, but not thermal hyperalgesia in dorsal column crush (DCC) rats. (A) Both CS-1 (DCC+CS-1, n=8) and fibronectin (DCC+FN, n=8) treatment resulted in a complete block of mechanical allodynia compared to rats treated with vehicle only (DCC+vehicle, n=10). (B) The hindpaw withdrawal latencies were not significantly influenced by DCC or fibronectin treatment. A thin line was added to indicate the pre-DCC baseline values as compared with later time points to showcase the development of allodynia.
Post-DCC locomotor function was evaluated by blinded observers for up to 4 months using the Basso-Beattie-Bresnahan (BBB) open-field locomotion test (Basso et al., 1995) to ensure the reliability of somatosensory testing. Neither forelimb or hindlimb motor scores were significantly affected by the C8 DCC or fibronectin treatment, which remained at a near perfect 20–21, values indicating normal locomotion, throughout the study period (data not shown). These results were supported by histological examination showing that our DCC largely injured only the ascending sensory fibers, but perhaps only minimally the underlying corticospinal tract (CST). As the rats appeared normal in behavior and gait, we were able to conclude that the increased hindpaw withdrawal thresholds resulted from the influence of either CS-1 or fibronectin treatment, but was not due to paralysis of the hindpaws.
Thermal sensitivity was also tested after intraspinal injection of fibronectin or CS-1 after DCC. Rats with DCC demonstrated no significant changes in hindpaw withdrawal latencies to radiant heat, which is indicative of thermal hyperalgesia. Furthermore, the treatment with fibronectin or CS-1 did not cause any significant changes in hindpaw withdrawal latencies over the 8-month observation period (Fig. 1B). Together, these behavioral assessments show that treatment with CS-1 or fibronectin led to decreased mechanical allodynia, but no significant changes in thermal hyperalgesia.
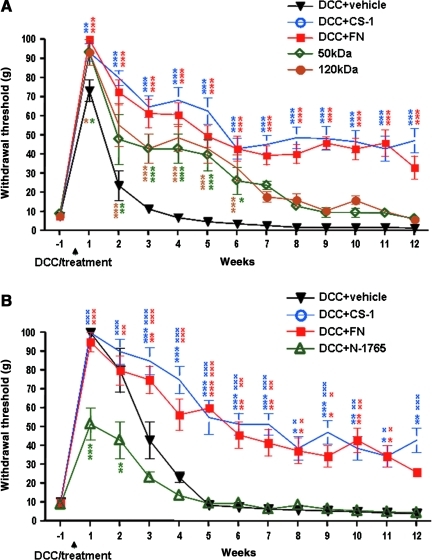
Two additional fibronectin fragments, a 50-kDa fragment (containing the binding site for integrin α5β1), and a 120-kDa fragment (containing the binding site for both integrin α4β1 and integrin α5β1), were further used to determine the active domain of fibronectin that maximally inhibited DCC-induced allodynia. Both the 50-kDa and 120-kDa fragments increased the hindpaw withdrawal threshold compared to the vehicle-treated group, but to a lesser extent compared to the CS-1- or fibronectin-treated groups (Fig. 2A). To investigate whether CS-1 and/or fibronectin engagement of integrin α4β1 is required for fibronectin-mediated inhibition of DCC-induced chronic pain development, N-1765, a selective tight-binding inhibitor of integrin α4β1, was applied immediately after DCC and mechanical allodynia was assessed on the hindpaws before and for 12 weeks after DCC. As before, the treatment with CS-1 or fibronectin maintained the hindpaw withdrawal thresholds significantly higher than their baseline and the vehicle-treated rats. However, N-1765 treatment alone significantly decreased the hindpaw withdrawal thresholds compared to vehicle-treated rats starting from 1 week after injury (Fig. 2B). Similar effects as N-1765 on the hindpaw withdrawal thresholds were seen when an integrin α4 function-blocking antibody was applied immediately after DCC (data not shown).
FIG. 2.
Different fibronectin fragments exerted different effects on allodynia following dorsal column crush (DCC), and the competitive inhibitor N-1765 blocked fibronectin's effects. (A) Treatment with additional two fibronectin fragments including a 50-kDa (n=3) and a 120-kDa fragment (n=3) also inhibited DCC-induced mechanical allodynia development, but to a lesser extent compared to the groups treated with connecting segment-1 (CS-1, n=5) or fibronectin (FN, n=4). (B) N-1765 treatment caused significant decreases in hindpaw withdrawal thresholds from 1 week after DCC (*versus vehicle-treated rats; xversus N-1765-treated rats).
Fibronectin attenuated DCC-induced increases in BSCB permeability and inflammation
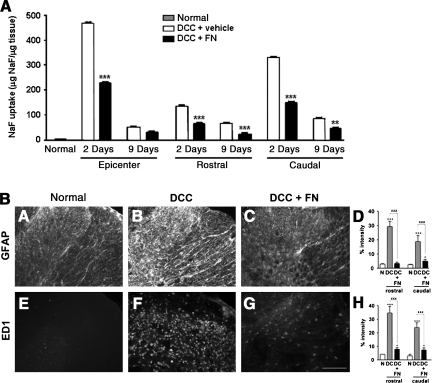
Altered BSCB permeability exposes the injured spinal cord to the toxic effects of infiltrating inflammatory cells and chemicals, which lead to secondary pathogenesis and chronic pain development after SCI (Mautes et al., 2000; Schlosshauer, 1993). We compared BSCB permeability changes in the vicinity of the lesion site in spinal cords of rats that had DCC with or without fibronectin injection. Extensive leakage of the fluorescent marker NaF from the circulation into the tissues of the spinal cord (an indicator of BSCB breakdown) was seen 2 days after DCC, especially in the lesion epicenter. Leakage declined to a lesser extent 9 days after DCC, although this was still more extensive than that which occurred in normal rats. However, treatment with fibronectin blocked DCC-induced increases in permeability in areas of the spinal cord surrounding the injury site at both 2 and 9 days after DCC (Fig. 3A). In addition, 3 days after injury, a glial fibrillary acidic protein (GFAP)-immunopositive region and an ED1-immunopositive region were seen surrounding and within the lesion, suggesting DCC-induced astrocyte and microglia/macrophage activation, respectively. However, treatment with fibronectin significantly decreased DCC-induced GFAP and ED1 immunoreactive intensity (Fig. 3B). These data suggest that the reduced presence of GFAP and ED1 by fibronectin treatment is attributable to the suppression of inflammatory responses after DCC.
FIG. 3.
Fibronectin treatment decreased dorsal column crush (DCC)-induced increases in blood–spinal cord barrier (BSCB) permeability, and the immunoreactivity of glial fibrillary acidic protein (GFAP) and ED1. (A) Rats with DCC were injected with fibronectin (DCC+FN, n=4). BSCB permeability to sodium fluorescein (NaF) was assessed in the tissues including at the epicenter (1-cm length of spinal cord surrounding the epicenter of lesion), and at the areas (1-cm-long) both rostral and caudal to the lesion of the spinal cord at 2 and 9 days after DCC. BSCB permeability changes are presented as the mean±standard deviation of NaF uptake in the tissues (μg NaF/μg tissue). Representative immunolabeling of GFAP (A–C, Normal, DCC, and DCC+FN), and ED1 (E–G) in the area caudal to the lesion demonstrated upregulation of GFAP (C) and ED1 (F), compared to normal rats (A and E) at 3 days after DCC. The graphs illustrate a significant increase after DCC (*versus normal rats), but a decrease with fibronectin treatment (xversus vehicle-treated DCC rats) in GFAP (D) and ED1 (H) immunoreactivity both rostral and caudal to the lesion (n=3 each group; N, normal rats; DC, vehicle-treated DCC rats; DC+FN, fibronectin-treated DCC rats; scale bar=100 mm).
Fibronectin blocks DCC-induced decreases in 5-HT levels in the superficial dorsal horn
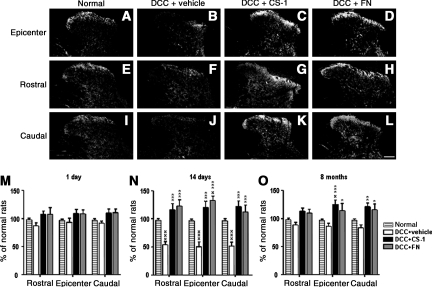
5-HT immunostaining in the normal spinal cord revealed immunoreactive fibers present predominantly in the superficial dorsal horn (laminae I and II), lamina X, and the ventral horn, with relatively little immunoreactivity observed in spinal white matter (Barritt et al., 2006; Figs. 4 and 5). We examined the influence of treatment on changes in the intensity of descending 5-HT projections to the dorsal horn after DCC, with and without concomitant application of CS-1 or fibronectin. Spinal cords from DCC rats with or without treatment were examined 1 day, 14 days, and 8 months post-DCC. Normal control rats displayed 5-HT immunoreactivity in the superficial dorsal horn (the pain processing region of the spinal cord). In the vehicle-treated DCC groups, 5-HT immunoreactivity was significantly decreased in the superficial dorsal horn by day 14 (Fig. 4B, F, and J). 5-HT immunoreactivity remained significantly decreased for as long as 8 months (Fig. 4). These morphological changes may have significance for the pathogenesis of chronic mechanical allodynia. Intraspinal one-time delivery of CS-1 or fibronectin to the DCC group resulted in 5-HT intensity that was markedly greater than that seen in vehicle-treated DCC rats from day 1 to 8 months post-DCC (Fig. 4). These results show that the spinal dorsal horn undergoes substantial structural plasticity not only at the lesion epicenter, but also both rostral and caudal to a DCC injury.
FIG. 4.
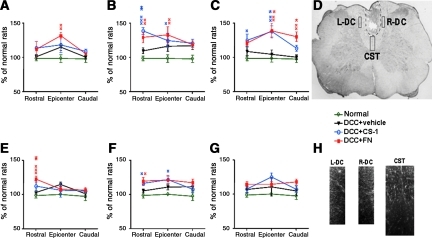
Serotonin (5-HT) immunoreactivity in the superficial dorsal horn was changed by dorsal column crush (DCC) and fibronectin treatment. Photomicrographs of 5-HT immunofluorescence from representative sections of spinal dorsal horn in normal rats (A, E, and I), and rats treated with vehicle (DCC+vehicle; B, F, and J), connecting segment-1 (CS-1; DCC+CS-1; C, G, and K), or fibronectin (DCC+FN; D, H, and L), at 14 days after DCC. The sections were collected from the lesion epicenter (A, B, C, and D) both rostral (E, F, G, and H), and caudal (I, J, K, and L) to the injury site (scale bar=100 mm). Quantification of spinal tissue 5-HT levels in epicenters and at the areas both rostral and caudal to the injury site collected from rats treated with vehicle, CS-1, or fibronectin (n=3 each group) at 1 day (M), 14 days (N), and 8 months (O) after DCC, and age-matched normal rats (xversus normal rats; *versus vehicle-treated DCC rats).
FIG. 5.
Fibronectin promotes plasticity of serotonin (5-HT) fibers in the dorsal white matter. After dorsal column crush (DCC) and vehicle treatment, 5-HT immunoreactivity was similar to that of controls, with a slight increase observed in the dorsal white matter in both the corticospinal tract (CST, top row; A, B, and C) and the dorsal column (DC, bottom row; E, F, and G), at 1 day (A and E), 14 days (B and F), and 8 months (C and G) after DCC. However, fibronectin treatment induced abundant sprouting of descending 5-HT fibers with numerous intensely-stained fibers apparent in the dorsal white matter (n=3 each group; xversus normal rats; *versus vehicle-treated DCC rats). (D) The lesion in the spinal cord caused by DCC is indicated by the dashed line. The boxed areas denote regions selected for quantification of 5-HT immunoreactivity in the CST (L-DC, left dorsal column; R-DC, right dorsal column). (H) A representative 5-HT-immunostaining image taken from the L-DC, R-DC, and CST.
Because fibronectin treatment was found to increase 5-HT immuoreactivity in the superficial dorsal horn, in an attempt to determine whether increased innervation of the gray matter might be attributable to increased fiber sparing, we examined 5-HT expression in the dorsal white matter. DCC animals treated with CS-1 (14 days) and fibronectin (1 day and 8 months) had significant-increases in 5-HT immunoreactivity in the dorsal white matter compared to controls (Fig. 5). Together these results show that fibronectin treatment resulted in significant increases in superficial dorsal horn as well as white matter 5-HT immunoreactivity, suggesting that less secondary damage may have occurred after fibronectin treatment. Sparing may also lead to enhanced sprouting from the remaining fibers.
5-HT activity is required for fibronectin effects on the inhibition of chronic SCI pain
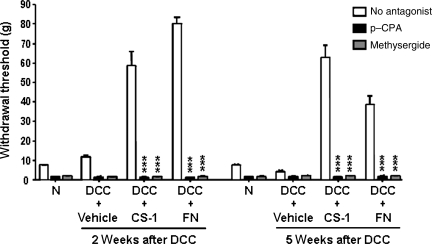
To test the possible role of 5-HT sparing/sprouting in CS-1- or fibronectin-mediated blockade of mechanical allodynia at 2 and 5 weeks after C8 DCC and fibronectin/CS-1 treatment, we depleted 5-HT with the 5-HT synthesis inhibitor para-chlorophenylalanine (p-CPA; Koe and Weissman, 1966). p-CPA administration resulted in a significant (98%) decrease in endogenous 5-HT levels throughout the spinal cord (data not shown). Depletion of spinal 5-HT via p-CPA resulted in increased mechanical allodynia (Fig. 6), to 1.6±0.49 g for CS-1, and 1.35±0.47 g for fibronectin, 2 weeks post-DCC, and 1.5±0.58 g for CS-1, and 1.75±0.5 g for fibronectin treatment 5 weeks post-DCC. In parallel experiments, methysergide was used to block 5-HT1/5-HT2 receptors non-selectively 2 or 5 weeks post-DCC. Methysergide significantly decreased the hindpaw withdrawal thresholds to similar levels as those observed with p-CPA (0.75±0.3 g for CS-1 and 1.35±0.47 g for fibronectin) 2 weeks post-DCC, and 5 weeks post-DCC (1.5±0.58 g for CS-1 and 2.0±1.4 g for fibronectin; Fig. 6). These results indicating methysergide inhibition of 5-HT activity suggest that CS-1 or fibronectin likely act to enhance the action of 5-HT at 5-HT1/5-HT2 receptors. The fact that the reduction in mechanical allodynia following CS-1 or fibronectin administration was attenuated both after methysergide and p-CPA injection supports the possibility that the increase in 5-HT immunoreactivity within spinal segments contributes, at least in part, to the anti-allodynia effects of both CS-1 and fibronectin.
FIG. 6.
The anti-allodynia effects of fibronectin are blocked by both para-chlorophenylalanine (p-CPA) and methysergide. Effects of p-CPA and methysergide on mechanical Allodynia were evaluated following treatment with vehicle, connecting segment-1 (CS-1), or fibronectin (FN), 2 or 5 weeks post-DCC. CS-1, fibronectin, or vehicle was administered immediately after DCC. p-CPA and methysergide were then administered at 2 or 5 weeks post-DCC, and mechanical allodynia was assessed (n=3 per group; *versus rats with no antagonist treatment).
Discussion
Our results demonstrate, for the first time, that a one-time intraspinal injection of fibronectin dramatically inhibits the development of chronic allodynia after spinal cord DCC over an 8-month observation period. In addition, fibronectin treatment attenuated BSCB breakdown and inflammatory extravasation in the areas surrounding the lesion. In turn, the reduction of 5-HT in the vicinity of the lesion in the superficial dorsal horn of the spinal cord was normalized after fibronectin treatment. The use of serotonergic antagonists resulted in a reversal of the therapeutic anti-allodynia effects of fibronectin. Thus we propose that the 5-HT system plays a major role in the modulation of the altered pain responses induced by DCC, and that fibronectin blocks hypersensitivity to pain by reducing inflammation-mediated axotomy or axonal dieback, and/or increases the sprouting of 5-HT fibers.
To ensure that hindpaw withdrawal of rats to stimuli reflected responses to pain (allodynia and/or hyperalgesia) rather than hyperreflexia, we were careful to record only paw withdrawals that were accompanied by complex behaviors mediated by the supraspinal pathways, such as paw licks, paw shakes, and startle. Indeed, the hindpaw withdrawal thresholds of vehicle-treated DCC rats became significantly smaller than their pre-DCC baseline values (Fig. 1A) at around 5 weeks post-DCC, which reveals that DCC is indeed a model for chronic SCI pain. The chronically evident mechanical allodynia induced by DCC is consistent with other previous reports, showing that mechanical allodynia following spinal hemisection is well developed 4 weeks post-section (Hulsebosch et al., 2000; Kim et al., 2005). A similar delay was seen in clinical reports that the onset of SCI pain is usually weeks to months after injury (Siddall et al., 1999,2003). Although we had measured both mechanical allodynia and thermal hyperalgesia, our data revealed that DCC induced only mechanical allodynia. This result is consistent with the interpretation that ascending spinal dorsal column projections are involved in the processing of mechanical rather than thermal nociception (Sun et al., 2001). Within the first day, an ipsilateral dorsal column lesion after peripheral nerve injury has been shown to block mechanical allodynia by means of large-diameter, myelinated fibers (Sun et al., 2001), which is consistent with our results showing insensitivity to stimuli 1 day after DCC. However, no data for time courses longer than 1 day in Sun's study can be used to compare to ours.
What are the mechanisms that cause a one-time intraspinal injection of fibronectin or particular fibronectin fragments to alleviate chronic pain? It is likely that integrin's presence in large amounts of exogenous fibronectin initiates numerous intracellular signaling pathways, leading to long-lasting activation of a wide variety of cell types in the lesion environment. The selected fibronectin fragments used to compare with the efficacy of full-length fibronectin included CS-1, a 50-kDa fragment, and a 120-kDa fragment. All fragments exerted inhibitory effects on the development of chronic DCC-induced allodynia, although CS-1 had the greatest effect, suggesting that the engagement of integrin α4β1 is especially important. The CS-1 fragment is much smaller than full-length fibronectin, which suggests that CS-1: (1) more easily diffuses away from the injected sites to exert its effects, (2) is more biologically stable because of fewer digestive sites for proteases, (3) is less likely to produce side effects because of the lack of binding sites for interaction, and (4) is more specific because the CS-1 fragment binds specifically to integrin α4β1, which is present on multiple cell types, including endothelium (Hamill 1987; Milner and Campbell, 2002), inflammatory cells (Bao et al., 2008), glia, and neurons (Lefcort et al., 1992; Su et al., 2008; Tomaselli et al., 1993; Werner et al., 2000). It is likely that the combined participation of all of these cell types upon fibronectin stimulation provides a more favorable microenviroment for later functional recovery (Kadoya et al., 2009).
Although the roles of integrins in inflammatory or neuropathic pain after SCI are under-explored, novel insights have emerged regarding the specific functional roles of integrins outside the CNS (Archelos et al., 1999; Jones, 1996). It has been reported that the intradermal injection of laminin and/or fibronectin selectively inhibits peripheral inflammatory hyperalgesia caused by prostaglandin E2 and epinephrine (Dina et al., 2004). These results strongly implicate specific integrins in the maintenance of hyperalgesia induced by peripheral nerve injuries, and our study suggests that similar phenomena may be occurring in the CNS.
Inflammation induced by SCI is known to contribute to enhanced nociceptive responses, including allodynia (Fitch and Silver, 2008; Hausmann, 2003). Studies have shown that thoracic SCI causes microglia to activate at multiple levels along the sensory pathways, which contributes to the maintenance of hyperexcitability of dorsal horn and thalamic somatosensory neurons, leading to chronic pain (Hains and Waxman, 2006; Zhao et al., 2007). Furthermore, microglia's attachment to fibronectin decreases proinflammatory cytokine IL-1β production in response to lipopolysaccharide treatment (Summers et al., 2009). This is consistent with our results that fibronectin treatment significantly decreased DCC-induced GFAP and ED1 immunoreactivity, which is typically enhanced in regions of BSCB breakdown (Fitch and Silver, 1997; Rhodes et al., 2006).
Altered BSCB permeability exposes the spinal cord to the toxic effects of infiltrating inflammatory cells and chemicals, which leads to secondary pathogenesis after SCI (Schlosshauer, 1993). The integrity of the cerebral microvasculature depends on the interaction between its component cells and the extracellular matrix, as well as reorganized cell–cell interactions. Fibronectin is an important extracellular matrix element of the endothelium, which becomes exposed when these tissues are injured (Hamill, 1987). Fibronectin and the integrins α4β1 and α5β1 are expressed by endothelium at high levels in the early post-natal CNS when angiogenesis is ongoing. They are present but reduced in the mature CNS, and their expression is highly upregulated during pathological conditions (Milner and Campbell, 2002). Taken together, these studies strongly suggest that specific integrins are important in regulating the function of blood vessels within the CNS. Our data show that fibronectin treatment resulted in a significant reduction of DCC-induced BSCB breakdown. This may be because fibronectin consists of multiple functional domains that can bind simultaneously and specifically to the microvasculature and various tissue elements, helping to maintain the integrity of the cerebral microvasculature by endothelium and its associated astrocytes (Milner and Campbell, 2002).
Spinal 5-HT remains the most intensively investigated transmitter in descending nociceptive control (Hains et al., 2003; Millan, 2002). The chronic deprivation of descending 5-HT inhibitory input after SCI, which can be reversed by administration of 5-HT (Tang et al., 2004), would leave nociceptive processing in the dorsal horn unregulated, contributing to the generation of chronic pain (Polistina et al., 1990; Zhang et al., 1993). After lesions in the CNS, the affected nerve fibers usually die back and cannot regenerate and reconnect to their original target (Deng et al., 2011; Lee et al., 2010). However, the remaining fibers can slowly form new collaterals and sprout into or beyond the lesion to compensate for certain lost functions (Kapfhammer, 1997). We speculated that a loss of inhibitory input would also occur rostral to the injury site, due to retrograde degeneration of injured descending axons, and that such a loss could be associated with the generation of chronic pain rostral to the injury. Indeed, our study demonstrated that in control animals, the 5-HT immunoreactivity seen in segments immediately rostral (C5–C6) to the injury site was significantly decreased after DCC. Although it is difficult to entirely rule out the possibility that the 5-HT increase seen in treated animals was a direct effect of fibronectin on raphe spinal axon sprouting, it is likely that the effect is indirect. 5-HT fibers are relatively resistant to inflammation-induced dieback, and have a propensity to sprout normally (Hawthorne et al., 2011). Fibronectin treatment may have lessened dieback even further, and induced enhanced plasticity of the descending serotonergic pathways. Alternatively, DCC may have caused the degeneration of collateral projections of the Aβ dorsal column sensory axons, which in turn might help induce collateral 5-HT sprouting (Wang et al., 1991), which is further stimulated by fibronectin treatment.
The long-lasting reduction of allodynia by fibronectin was lost following both methysergide and p-CPA administration, suggesting that reduced allodynia following intraspinal fibronectin may be mediated via 5-HT-induced activation of 5-HT1/5-HT2 receptors. The 5-HT1 receptor is the most abundant 5-HT receptor found in the superficial dorsal horn (Marlier et al., 1991; Zemlan et al., 1988), and it exerts anti-nociceptive effects (Sanchez et al., 1995). Taken together our results suggest that increased 5-HT fiber density immediately rostral and caudal to the lesion site could help reduce mechanical allodynia via actions at 5-HT1/5-HT2 receptors. This is the first demonstration that fibronectin can induce plasticity within the spinal cord after SCI, and suggests compensatory sprouting of descending projections as a mechanism underlying the inhibition of mechanical allodynia.
Acknowledgments
We thank Jianguo Cheng, Lyle Fox, Jun Shen, Sarah A. Busch, and Carine Savarin for technical assistance, and Christopher Nelson for editorial assistance. We thank Mohamed Naguib for constructive criticism. We also thank Sue Craig from the University of Manchester, U.K. for providing the 50-kDa fibronectin fragment. This work was supported by the grants from the Cleveland Clinic Foundation to C.-Y. Lin, the National Institutes of Health/National Institute for Neurological Diseases and Stroke (NIH/NINDS) no. NS069765 to Y.-S. Lee, and NIH/NINDS no. NS25713 to J. Silver.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander J.K. Popovich P.G. Neuroinflammation in spinal cord injury: therapeutic targets for neuroprotection and regeneration. Prog. Brain Res. 2009;175:125–137. doi: 10.1016/S0079-6123(09)17508-8. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Andrew S.M. Edwards B.D. Chalmers R.J. O'Driscoll J.B. A quantitative immunohistochemical study of the expression of integrins by nerves in psoriatic and normal skin. Br. J. Dermatol. 1992;127:359–364. doi: 10.1111/j.1365-2133.1992.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Archelos J.J. Previtali S.C. Hartung H.P. The role of integrins in immune-mediated diseases of the nervous system. Trends Neurosci. 1999;22:30–38. doi: 10.1016/s0166-2236(98)01287-9. [DOI] [PubMed] [Google Scholar]
- Baastrup C. Finnerup N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22:455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Bao F. Chen Y. Schneider K.A. Weaver L.C. An integrin inhibiting molecule decreases oxidative damage and improves neurological function after spinal cord injury. Exp. Neurol. 2008;214:160–167. doi: 10.1016/j.expneurol.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Bareyre F.M. Kerschensteiner M. Raineteau O. Mettenleiter T.C. Weinmann O. Schwab M.E. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barritt A.W. Davies M. Marchand F. Hartley R. Grist J. Yip P. McMahon S.B. Bradbury E.J. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso D.M. Beattie M.S. Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Busch S.A. Horn K.P. Silver D.J. Silver J. Overcoming macrophage-mediated axonal dieback following CNS injury. J. Neurosci. 2009;29:9967–9976. doi: 10.1523/JNEUROSCI.1151-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M.D. Hulsebosch C.E. Chronic central pain after spinal cord injury. J. Neurotrauma. 1997;14:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- Christensen M.D. Everhart A.W. Pickelman J.T. Hulsebosch C.E. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;68:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- Deng L.X. Hu J. Liu N. Wang X. Smith G.M. Wen X. Xu X.M. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp. Neurol. 2011;229:238–250. doi: 10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina O.A. Parada C.A. Yeh J. Chen X. McCarter G.C. Levine J.D. Integrin signaling in inflammatory and neuropathic pain in the rat. Eur. J. Neurosci. 2004;19:634–642. doi: 10.1111/j.1460-9568.2004.03169.x. [DOI] [PubMed] [Google Scholar]
- Fabis M.J. Phares T.W. Kean R.B. Koprowski H. Hooper D.C. Blood-brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc. Natl. Acad. Sci. USA. 2008;105:15511–15516. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup N.B. A review of central neuropathic pain states. Curr. Opin. Anaesthesiol. 2008;21:586–589. doi: 10.1097/ACO.0b013e32830a4c11. [DOI] [PubMed] [Google Scholar]
- Fitch M. Doler C. Combs C. Landreth G. Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch M.T. Silver J. Activated marcrophages and the blood brain barrier: inflammation after CNS injury leads to increase in putative inhibitory molecules. Exp. Neurol. 1997;148:587–603. doi: 10.1006/exnr.1997.6701. [DOI] [PubMed] [Google Scholar]
- Fitch M.T. Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J.C. Bao F. Chen Y. Hamilton E.F. Relton J.K. Weaver L.C. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp. Neurol. 2008;214:147–159. doi: 10.1016/j.expneurol.2008.04.024. [DOI] [PubMed] [Google Scholar]
- Fouad K. Krajacic A. Tetzlaff W. Spinal cord injury and plasticity: opportunities and challenges. Brain Res. Bull. 2011;84:337–342. doi: 10.1016/j.brainresbull.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Hadley S.D. Walker P.D. Goshgarian H.G. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp. Neurol. 1999;160:433–445. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- Hains B.C. Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains B.C. Willis W.D. Hulsebosch C.E. Serotonin receptors 5-HT1A and 5-HT3 reduce hyperexcitability of dorsal horn neurons after chronic spinal cord hemisection injury in rat. Exp. Brain Res. 2003;149:174–186. doi: 10.1007/s00221-002-1352-x. [DOI] [PubMed] [Google Scholar]
- Hamill R.J. Role of fibronectin in infective endocarditis. Rev. Infect. Dis. 1987;9(Suppl. 4):S360–S371. doi: 10.1093/clinids/9.supplement_4.s360. [DOI] [PubMed] [Google Scholar]
- Hao J.X. Xu X.J. Aldskogius H. Seiger A. Wiesenfeld-Hallin Z. Photochemically induced transient spinal ischemia induces behavioral hypersensitivity to mechanical and cold stimuli, but not to noxious-heat stimuli, in the rat. Exp. Neurol. 1992;118:187–194. doi: 10.1016/0014-4886(92)90035-o. [DOI] [PubMed] [Google Scholar]
- Hausmann O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- Hawthorne A.L. Hu H. Kundu B. Steinmetz M.P. Wylie C.J. Deneris E.S. Silver J. The unusual response of serotonergic neurons after CNS injury: Lack of axonal dieback and enhanced sprouting within the inhibitory environment of the glial scar. J. Neurosci. 2011;31:5605–5616. doi: 10.1523/JNEUROSCI.6663-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. Hains B.C. Crown E.D. Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60:202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C.E. Xu G.Y. Perez-Polo J.R. Westlund K.N. Taylor C.P. McAdoo D.J. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J. Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Jefferson S.C. Tester N.J. Howland D.R. Chondroitinase ABC promotes recovery of adaptive limb movements and enhances axonal growth caudal to a spinal hemisection. J. Neurosci. 2011;31:5710–5720. doi: 10.1523/JNEUROSCI.4459-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen T.S. Madsen C.S. Finnerup N.B. Pharmacology and treatment of neuropathic pain. Curr. Opin. Neurol. 2009;22:467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- Jones L.S. Integrins: possible functions in the adult CNS. Trends Neurosci. 1996;19:68–72. doi: 10.1016/0166-2236(96)89623-8. [DOI] [PubMed] [Google Scholar]
- Kadoya K. Tsukada S. Lu P. Coppola G. Geschwind D. Filbin M.T. Blesch A. Tuszynski M.H. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer J.P. Restriction of plastic fiber growth after lesions by central nervous system myelin-associated neurite growth inhibitors. Adv. Neurol. 1997;73:7–27. [PubMed] [Google Scholar]
- Kim J. Back S.K. Yoon Y.W. Hong S.K. Na H.S. Dorsal column lesion reduces mechanical allodynia in the induction, but not the maintenance, phase in spinal hemisected rats. Neurosci. Lett. 2005;379:218–222. doi: 10.1016/j.neulet.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Koe B.K. Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J. Pharmacol. Exp. Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- Lee J.K. Chow R. Xie F. Chow S.Y. Tolentino K.E. Zheng B. Combined genetic attenuation of myelin and semaphorin-mediated growth inhibition is insufficient to promote serotonergic axon regeneration. J. Neurosci. 2010;30:10899–10904. doi: 10.1523/JNEUROSCI.2269-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefcort F. Venstrom K. McDonald J.A. Reichardt L.F. Regulation of expression of fibronectin and its receptor, alpha 5 beta 1, during development and regeneration of peripheral nerve. Development. 1992;116:767–782. doi: 10.1242/dev.116.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B.H. Carman C.V. Springer T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier L. Teilhac J.R. Cerruti C. Privat A. Autoradiographic mapping of 5-HT1, 5-HT1A, 5-HT1B and 5-HT2 receptors in the rat spinal cord. Brain Res. 1991;550:15–23. doi: 10.1016/0006-8993(91)90400-p. [DOI] [PubMed] [Google Scholar]
- Mautes A.E. Weinzierl M.R. Donovan F. Noble L.J. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys. Ther. 2000;80:673–687. [PubMed] [Google Scholar]
- Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Mills C.D. Grady J.J. Hulsebosch C.E. Changes in exploratory behavior as a measure of chronic central pain following spinal cord injury. J. Neurotrauma. 2001;18:1091–1105. doi: 10.1089/08977150152693773. [DOI] [PubMed] [Google Scholar]
- Milner R. Campbell I.L. The integrin family of cell adhesion molecules has multiple functions within the CNS. J. Neurosci. Res. 2002;69:286–291. doi: 10.1002/jnr.10321. [DOI] [PubMed] [Google Scholar]
- Ochi T. Ohkubo Y. Mutoh S. The spinal antinociceptive effect of kyotorphin in mice: involvement of the descending noradrenergic and serotonergic systems. Neurosci. Lett. 2002;329:193–196. doi: 10.1016/s0304-3940(02)00647-x. [DOI] [PubMed] [Google Scholar]
- Patterson J.T. Chung K. Coggeshall R.E. Further evidence for the existence of long ascending unmyelinated primary afferent fibers within the dorsal funiculus: effects of capsaicin. Pain. 1992;49:117–120. doi: 10.1016/0304-3959(92)90197-J. [DOI] [PubMed] [Google Scholar]
- Polistina D.C. Murray M. Goldberger M.E. Plasticity of dorsal root and descending serotoninergic projections after partial deafferentation of the adult rat spinal cord. J. Comp. Neurol. 1990;299:349–363. doi: 10.1002/cne.902990307. [DOI] [PubMed] [Google Scholar]
- Rhodes K.E. Raivich G. Fawcett J.W. The injury response of oligodendrocyte precursor cells is induced by platelets, macrophages and inflammation-associated cytokines. Neuroscience. 2006;140:87–100. doi: 10.1016/j.neuroscience.2006.01.055. [DOI] [PubMed] [Google Scholar]
- Sanchez A. Niedbala B. Feria M. Modulation of neuropathic pain in rats by intrathecally injected serotonergic agonists. Neuroreport. 1995;6:2585–2588. doi: 10.1097/00001756-199512150-00032. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol. Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Schlosshauer B. The blood-brain barrier: morphology, molecules, and neurothelin. Bioessays. 1993;15:341–346. doi: 10.1002/bies.950150508. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. McClelland J.M. Rutkowski S.B. Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103:249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Siddall P.J. Taylor D.A. McClelland J.M. Rutkowski S.B. Cousins M.J. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81:187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Su L. Lv X. Miao J. Integrin beta 4 in neural cells. Neuromolecular Med. 2008;10:316–321. doi: 10.1007/s12017-008-8042-1. [DOI] [PubMed] [Google Scholar]
- Summers L. Kielty C. Pinteaux E. Adhesion to fibronectin regulates interleukin-1 beta expression in microglial cells. Mol. Cell Neurosci. 2009;41:148–155. doi: 10.1016/j.mcn.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Sun H. Ren K. Zhong C.M. Ossipov M.H. Malan T.P. Lai J. Porreca F. Nerve injury-induced tactile allodynia is mediated via ascending spinal dorsal column projections. Pain. 2001;90:105–111. doi: 10.1016/s0304-3959(00)00392-4. [DOI] [PubMed] [Google Scholar]
- Tang X.Q. Tanelian D.L. Smith G.M. Semaphorin 3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J. Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli K.J. Doherty P. Emmett C.J. Damsky C.H. Walsh F.S. Reichardt L.F. Expression of beta 1 integrins in sensory neurons of the dorsal root ganglion and their functions in neurite outgrowth on two laminin isoforms. J. Neurosci. 1993;13:4880–4888. doi: 10.1523/JNEUROSCI.13-11-04880.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers E.R. Cousins M.J. Neuropathic orofacial pain part 1—prevalence and pathophysiology. Aust. Endod. J. 2000;26:19–26. doi: 10.1111/j.1747-4477.2000.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Vierck C.J., Jr. Light A.R. Effects of combined hemotoxic and anterolateral spinal lesions on nociceptive sensitivity. Pain. 1999;83:447–457. doi: 10.1016/S0304-3959(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Wang S.D. Goldberger M.E. Murray M. Plasticity of spinal systems after unilateral lumbosacral dorsal rhizotomy in the adult rat. J. Comp. Neurol. 1991;304:555–568. doi: 10.1002/cne.903040405. [DOI] [PubMed] [Google Scholar]
- Werner A. Willern M. Jones L.L. Dreutzberg G.W. Mayer Y. Raivich G. Impaired axonal regeneration in a7 integrin-deficient mice. J. Neurosci. 2000;20:1822–1830. doi: 10.1523/JNEUROSCI.20-05-01822.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W.D., Jr. Westlund K.N. The role of the dorsal column pathway in visceral nociception. Curr. Pain Headache Rep. 2001;5:20–26. doi: 10.1007/s11916-001-0006-1. [DOI] [PubMed] [Google Scholar]
- Wolfe S.Q. Garg M. Cumberbatch N.M. Furst C. Martinez M. Hernandez M. Reimers R. Berrocal Y. Gómez-Marín O. Eaton M.J. Optimizing the transplant dose of a human neuronal cell line graft to treat SCI pain in the rat. Neurosci. Lett. 2007;414:121–125. doi: 10.1016/j.neulet.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Zemlan F.P. Behbehani M.M. Murphy R.M. Serotonin receptor subtypes and the modulation of pain transmission. Prog. Brain Res. 1988;77:349–355. doi: 10.1016/s0079-6123(08)62801-0. [DOI] [PubMed] [Google Scholar]
- Zhang B. Goldberger M.E. Murray M. Proliferation of SP- and 5HT-containing terminals in lamina II of rat spinal cord following dorsal rhizotomy: quantitative EM-immunocytochemical studies. Exp. Neurol. 1993;123:51–63. doi: 10.1006/exnr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Zhao P. Waxman S.G. Hains B.C. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J. Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]