Fig. 1.
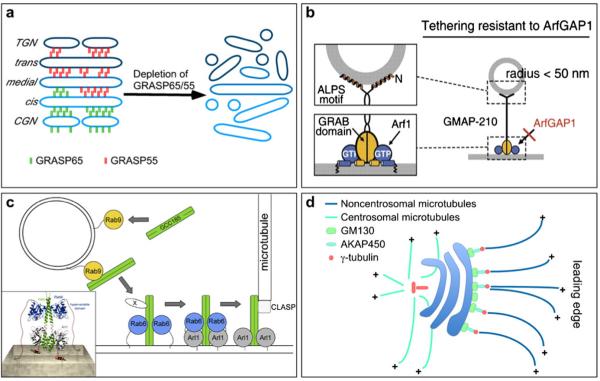
A schematic of new features of Golgi matrix proteins. a GRASP65 and GRASP55, two homologous proteins localized to the cis and medial-to-trans Golgi cisternae, respectively, mediate cisternal stacking by forming cell cycle-regulated oligomers. The depletion of GRASP65 and GRASP55 causes disassembly of the Golgi stacks. b GMAP-210, a cis-Golgi localized golgin, is composed of a 38 aa N-terminal ALPS motif, a C-terminal GRAB domain and six coiled-coil domains. The ALPS motif binds to highly curved membranes, whereas the GRAB domain binds to a flat membrane, which is stabilized by Arf-GTP. This novel structure suggests that GMAP-210 asymmetrically tethers the flat and curved membranes. Reprinted and modified from Drin et al. (2008) with permission from the journal publisher. c A model of GCC185 transferring from a vesicle to the Golgi membrane. Left A cytosolic GCC185 dimer is first recruited to the Rab9-bearing vesicles and interacts with Golgi-bound GCC185 via an unknown protein (X), which is followed by SNARE pairing (not shown). Middle GCC185 is targeted to the Golgi membranes by an interaction with Rab6, which may also promote Arl1 binding to the GRIP domain of GCC185. Right Golgi-anchored GCC185 interacts with CLASP, which promotes microtubule assembly at the Golgi apparatus. Inset: the model of Rab6 and Arl1 bound to the GCC185 Rab-binding domain (RBD) and GRIP domain, respectively. Reprinted and modified from Burguete et al. 008) with permission from the journal publisher. d GM130, a cis Golgi-localized coiled-coil protein originally found in the GM130-p115-giantin complex that is involved in ER-to-Golgi trafficking, interacts with AKAP450, which recruits γ-tubulin to the Golgi apparatus and participates in the organization of non-centrosomal microtubules that are critical for leading edge transport and directed migration (Efimov et al. 2007; Rivero et al. 2009)