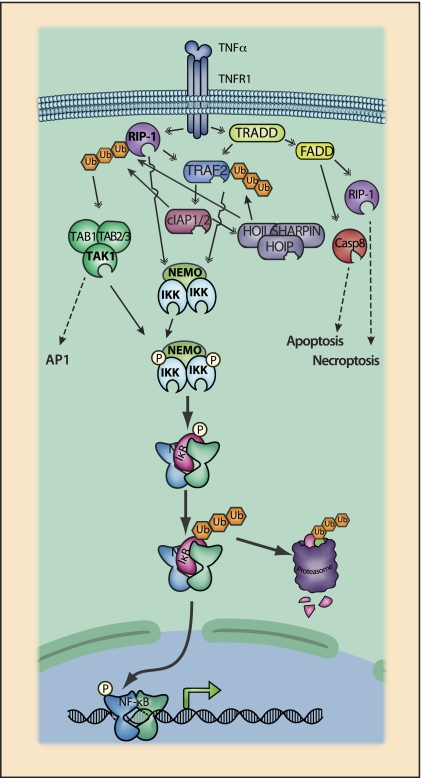
Figure 3.
TNFR1 signaling to NF-κB. TNFR1 activates multiple signaling pathways, including NF-κB, AP-1, and the apoptosis and necroptosis cell death pathways. TNF-induced activation of NF-κB is mediated by a series of intermediary adapters. The cytoplasmic tail of TNFR1 exhibits several protein-binding domains, most notably a death domain (DD) that mediates signaling events following TNF binding. Signaling events are partially organized by subcellular compartmentalization of receptor complexes, and the TNFR cytoplasmic tail contains adapter protein-binding motifs that direct trafficking following TNF binding (Schutze and Schneider-Brachert 2009). Upon ligand binding, the DD of TNFR1 binds TRADD (TNFR-associated protein with a DD) and the DD-containing kinase RIP1 (Box 3). Mechanisms coordinating binding between the DDs of TRADD, RIP1, and TNFR1 are not fully established. Nevertheless, it is clear that each of these DD-containing proteins are capable of binding to other DD-containing proteins (Wajant and Scheurich 2011). TRADD also provides an assembly platform for recruitment of another DD adapter protein, FADD (Fas receptor-associated DD). TNFR1 lacks a TRAF interaction motif, and TRAF recruitment is thus also dependent on TRADD, which has a TRAF-binding domain. Although RIP1 also has a TRAF-binding domain and may contribute to TRAF2 recruitment under some circumstances (Pobezinskaya et al. 2008), it is generally thought that TRAF2 recruitment is primarily dependent on TRADD (Chen et al. 2008; Ermolaeva et al. 2008; Pobezinskaya et al. 2008). TRAF2 recruits cIAP1 and cIAP2 (Box 2), which are essential for IKK activation (Mahoney et al. 2008; Varfolomeev et al. 2007; Vince et al. 2007). The cIAPs can function as E3 ubiquitin ligases and are also responsible for the recruitment of the linear ubiquitin chain assembly complex, which is required for efficient activation of IKK and JNK pathways (LUBAC [linear ubiquitin assembly complex]) (Box 4; Haas et al. 2009; Rahighi et al. 2009; Tokunaga et al. 2009, 2011; Gerlach et al. 2011; Ikeda et al. 2011). RIP1 and TRAF2 cooperate in the recruitment of the TAK1 and IKK kinase complexes, leading to IKK activation and activation of NF-κB.