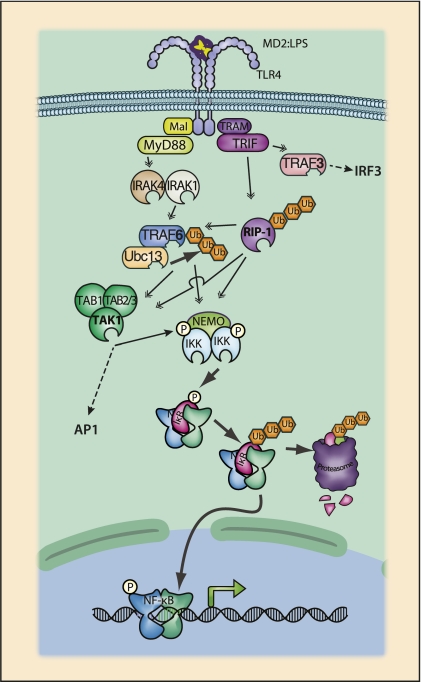
Figure 4.
TLR4 signaling to NF-κB. Both TLR and IL-1R receptor families are defined by the presence of cytoplasmic TIR (Toll IL-1R) domains. Upon ligand binding, TIR domains mediate the recruitment of TIR-containing adapter proteins such as MyD88, TRIF, Mal, or TRAM (Yamamoto et al. 2004a). TLR4, which responds to bacterial lipopolysaccharide, has a complex bifurcating signaling scheme. MyD88 is the prototypical TIR adapter and is used in all characterized TLR signaling pathways, with the exception of TLR3. TLR4 recognizes LPS bound to either LPS-binding protein or MD2, and signaling is also dependent on the glycoprotein CD14. Formation of a complex between LPS and TLR4:MD2:CD14 results in the homodimerization of TLR4 and recruitment of the TIR-containing adapters Mal and TRAM. Mal serves as an adapter to recruit MyD88 to TLR4, while TRAM is an adapter between TLR4 and TRIF. Following recruitment to the receptor complex, dimerized MyD88 recruits IL-1R-associated kinases-4 (IRAK-4) through the DD of MyD88 and IRAK-4. IRAK-4 recruits IRAK-1, and the IRAK-1:IRAK-4 complex is responsible for binding to TRAF6. TRAF6, in turn, recruits the TAK1 and IKK complexes, leading to activation of NF-κB. TRIF, recruited by TRAM, predominantly activates the interferon pathway through an N-terminal TRAF3-binding motif. TRAF3 recruits the IKK family members IKKɛ and TBK1, which phosphorylate IRF3, leading to the induction of type I interferons. TRIF may also induce NF-κB activation through a C-terminal RHIM (RIP homology interaction motif) domain capable of recruiting RIP1 and also the IKK complex (Cusson-Hermance et al. 2005).