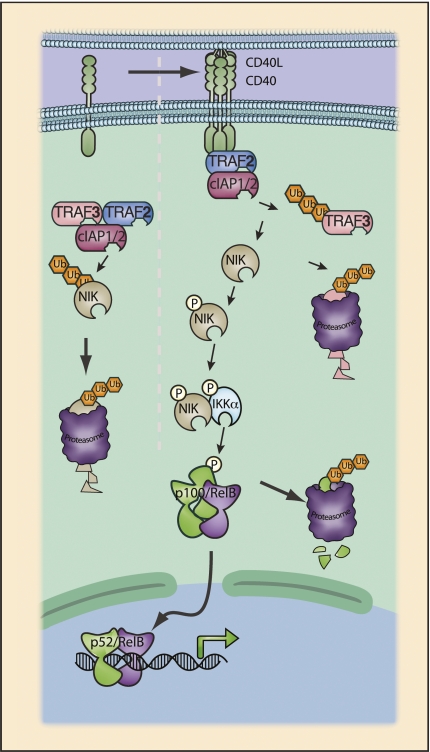
Figure 6.
CD40 signaling to the alternative NF-κB pathway. CD40 possesses multiple TRAF interaction motifs, and binding of CD40L to CD40 triggers direct binding to multiple TRAF proteins. Noncanonical NF-κB activation requires the NF-κB-inducing kinase (NIK). NIK is constitutively active; thus, the noncanonical pathway is activated through the post-translational regulation of NIK protein levels. In the steady state, NIK is subject to constitutive ubiquitination by TRAF3 and consequent degradation. Upon CD40 ligation, TRAF2 is recruited to the receptor and, in conjunction with cIAP, targets TRAF3 for proteasomal degradation (Liao et al. 2004). CD40 ligation may also promote NIK stabilization through an “allosteric model” in which binding of NIK and binding of CD40 by TRAF proteins are mutually exclusive events (Sanjo et al. 2010). Thus, upon TRAF binding to CD40, NIK is displaced from the TRAF2:TRAF3 complex. As a result of displacement of NIK and the loss of TRAF3, active NIK accumulates. NIK binds and phosphorylates IKKα, leading to activation of IKKα kinase activity. Phosphorylation of p100 C-terminal serine residues by IKKα results in p100 ubiquitination by the SCFβTRCP complex and proteasomal processing to p52. Activation of the noncanonical pathway has also been well characterized for LTβR, BAFFR, and RANK.