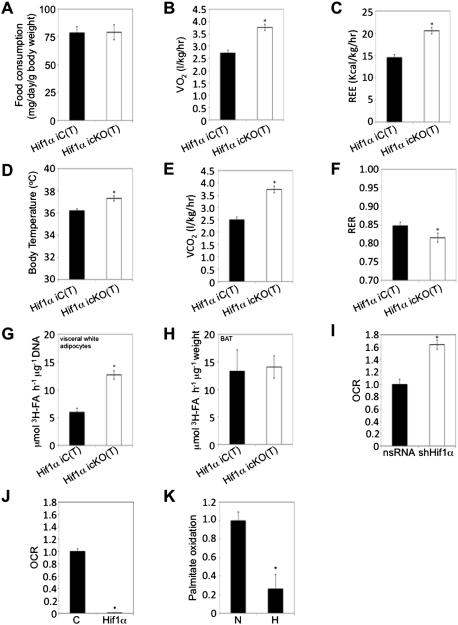
Figure 2.
Visceral adipose Hif1α inactivation promotes fatty acid β-oxidation and systemic energy expenditure. (A) Individually housed Hif1α iC(T) (n = 10) and Hif1α icKO(T) (n = 12) mice maintained on a HFD/HFD protocol were assessed for food intake over a period of 3 wk (between weeks 12–15 post-tamoxifen administration). Data are mean ± SEM of values from each group. (B–F) Hif1α iC(T) and Hif1α icKO(T) mice subjected to the HFD/HFD protocol were placed in metabolic cages at 15 wk post-Hif1α inactivation. Resting O2 consumption (B), energy expenditure (C), body temperature (D), CO2 expiration (E), and RER (F) measurements are shown. (*) P < 0.01 versus Hif1α iC(T) HFD/HFD. Data are mean ± SEM of values from six mice per group. (G,H) Primary visceral white adipocytes (G) and BAT explants (H) isolated from Hif1α iC(T) and Hif1α icKO(T) mice maintained on the HFD/HFD protocol were assessed for palmitate oxidation. (*) P < 0.05 veruss Hif1α iC(T) HFD/HFD. Data are mean ± SEM of values from four mice per group. (I,J) 3T3-L1-derived adipocytes were infected with shHif1α-encoding viruses or the corresponding nsRNA control (I), empty (mock, C), or Hif1α-encoding (J) virus and assessed for oleate-induced oxygen consumption rate (OCR) as a measure of fatty acid β-oxidation using the Seahorse Bioscience 24XF extracellular flux analyzer. (*) P < 0.01 compared with control, set at 1.0. Data are mean ± SEM of values from each group. (K) Differentiated 3T3-L1 adipocytes were cultured in normoxia (N, 20% O2) or hypoxia (H, 3% O2) and assessed for palmitate oxidation. (*) P < 0.01 compared with normoxia control (N), set at 1.0. Data are mean ± SEM of values from each group.