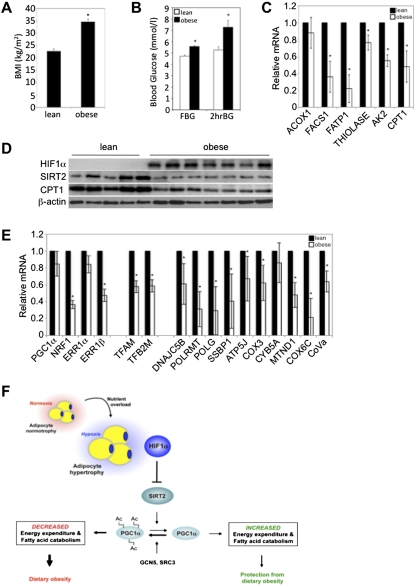
Figure 6.
Sirt2 is down-regulated in human obesity. (A) Average BMI of subjects within the lean (n = 9) and obese (n = 9) groups, respectively. (*) P < 0.01 versus lean subjects. Data are mean ± SEM of values from each group. (B) Fasting (FBG) and 2-h oral GTT (2hrBG) blood glucose indices of the lean (n = 9) and obese (n = 9) groups are shown. (*) P < 0.05 versus lean subjects. Data are mean ± SEM of values from each group. (C) Gene expression profiling of visceral WAT biopsies of lean and obese subjects for components of FAO. All values were normalized internally to 18S RNA expression and to the lean samples, respectively. (*) P < 0.05 versus lean subjects. Data are mean ± SEM of values from the respective groups. (D) Visceral WAT biopsies of human lean or obese subjects were assessed for HIF1α, SIRT2, and CPT1 protein expression. β-Actin served as a loading control. (E) Gene expression profiling of visceral WAT biopsies of lean and obese subjects for regulators mitochondrial biogenesis and of mitochondrial complex and electron transport chain proteins. All values were normalized internally to 18S RNA expression and to the lean samples, respectively. (*) P < 0.05 versus lean subjects. Data are mean ± SEM of values from the respective groups. (F) Schematic representation of the transcriptional and enzymatic coregulation of SIRT2 by HIF1α. Nutrient overload-induced adipose expansion augments intra-adipose hypoxia, leading to adipocyte HIF1α accumulation. HIF1α represses SIRT2 transcription via interaction at a cross-species conserved HRE on the SIRT2 promoter. The repression of SIRT2 activity by HIF1α is predicted to support the maintenance of general control of amino acid synthesis, yeast, homolog-like 2 (GCN5/KAT2A)-mediated, and/or steroid receptor coactivator protein 3 (SRC3/NCOA3)-mediated Pgc1α hyperacetylation and its consequent inactivation, culminating in the maintenance of lipid anabolism and pathological adipose expansion. For additional details, see the text.