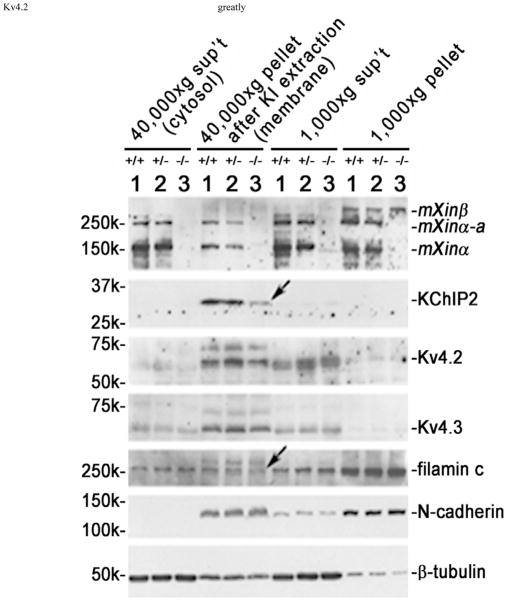
Figure 9.
Juvenile mXinα-null ventricles exhibited significant decreases in both membrane-associated KChIP2 and filamin proteins (indicated by arrows). Total ventricular extracts were prepared as described under Materials and Methods in a low salt buffer, and spinned at 1,000×g centrifugation. The 1,000×g pellet contained nuclei, debris and low salt-resistant protein aggregates. The membrane vesicles, myofibrils and organelles in the 1,000×g supernatant (sup’t) were collected by 40,000×g centrifugation. After extracted with 6 M KI to remove myofibrils, the 40,000×g pellet and sup’t were termed “membrane” and “cytosolic” fractions, respectively. Western blot analysis was performed on these fractions prepared from ventricles of wild type (+/+, lane 1), heterozygous (+/−, lane 2) and homozygous (−/−, lane 3) littermates at 1 month of age. An equivalent volume from each fraction was loaded onto the SDS-PAGE gel, except 10 times concentrated sample of membrane fraction was used, and analyzed by Western blot with antibodies including U1013, anti-KChIP2, anti-Kv4.2, anti-Kv4.3, anti-filamin, anti-N-cadherin and anti-β-tubulin. The majority of Kv4.2 and Kv4.3, α-subunits, of ITO channel together with most of N-cadherin were detected in the membrane fraction, whereas most of β-tubulin was found in the cytosolic fraction.