Abstract
Increasing evidence suggests that Mannan-binding lectin (MBL), the initial factor of the lectin pathway of complement, plays a role in cardiovascular diseases, i.e. inversely associated with risk of myocardial infarction (MI). In the present case, a patient with MBL deficiency underwent coronary artery bypass grafting (CABG) after an acute MI with underlining chronic lymphocytic leukemia (CLL). Post-operatively, the patient had a cerebral vascular accident and eventually expired. Analysis of his blood samples from pre-, intra-, and post-operative periods showed that MBL levels abruptly increased post-operatively. We hypothesize that the post-operative increase of MBL in the patient with pre-existing MBL deficiency may contribute to systemic inflammation, causing a detrimental effect after cardiac surgery.
1. Introduction
Circulating MBL is synthesized by liver, monocytes, and leukocytes, and functions as the initial factor in the lectin pathway of complement [1]. MBL binds carbohydrates pattern on microorganisms or altered self targets, and subsequently activates MBL-associated serine proteases (MASPs). MASPs further activate downstream complement proteins, i.e. C4 and C2, and eventually lead to the destruction of the targets. In addition, activation of complement cascade releases cleaved fragments such as C3a and C5a, potent chemoattractants for inflammatory cells.
Increasing evidence has suggested that MBL plays an important role in cardiovascular diseases. MBL is found to be deposited throughout the ischemic area at risk in an animal myocardial model [2]. Clinical studies showed that the level of MBL is inversely associated with the risk of myocardial infarction [3], and low MBL was correlated with the development of acute rejection after heart transplantation [4]. MBL deficiency is reported to be associated with atherosclerosis [5, 6], arterial thrombosis in systemic lupus erythematosus [7], venous bypass graft occlusions in patients with coronary heart disease [8], and a high rate of early restenosis [9].
2. Case Report
A 75 year-old male patient with a past medical history of CLL, congestive heart failure (CHF), hypertension, cirrhosis (Child’s class B), and prostate surgery presented to the emergency room of a community hospital with chest pain and dyspnea. He was admitted for non-ST elevated MI and CHF exacerbation. Coronary angiography demonstrated severe left main coronary artery disease with an ejection fraction of 25% (Table 1). The patient had marked decrease in level of responsiveness during and immediately after the cardiac catheterization, and was transferred to SUNY-Downstate Medical Center on Intra Aortic Balloon Pump for emergent CABG (LIMA to LAD, SVG to OM, distal RCA). He was on the cardio-pulmonary bypass (CPB) machine for 109 minutes and aortic cross-clamping for 79 minutes. The patient returned to the operation room the same evening for re-exploration and evacuation of clots. Post-operatively, the patient never fully regained consciousness and continued to require respiratory assistance. Additionally, he had decreased motion over his left side. Head CT demonstrated an area of old infarct over the right fronto-parietal and parieto-occiptal regions.
Table 1.
Demographic and baseline data
| Age | 75 |
| Gender | Male |
| Body Mass Index | 28.5 |
| Smoker | Yes |
| Diabetes | No |
| Hypercholesterolemia | Yes |
| Hypertension | Yes |
| Diagnosis | Non-ST Elevated Myocardial Infarction |
| Race / Ethnics | Hispanic |
| WBC (admission) | 32.5 × 106 /ul (normal range: 4.8~10.8 × 106 /ul) |
| Bone Marrow Biopsy | > 80% of lymphocytes are B cell (CD20+) |
| Immunophenotyping by flow cytometry | B-CLL/ SLL: CD19+, CD5+, CD20+, CD23+, CD38, kappa light chain restriction |
| Cardiac Catheterization | Left Anterior Descending artery: 75% occluded Obtuse Marginal artery (first): 75% occluded |
Ten days later, the patient began to experience abdominal distention and hypotension not responsive to fluid boluses. Over the ensuing few hours, he experienced multiple runs of supra-ventricular tachycardia requiring adenosine, vasopressin, lidocaine and electrical cardioversion. Transthoracic echocardiography showed poor acoustic window secondary to distended stomach, but no evidence of tamponade. There was no evidence of free air on chest or abdominal x-rays. The patient’s blood pressure began to trend downward necessitating increasing pressor support until eventually he became asystolic. ACLS protocol was followed and the patient was unable to be revived. Post-mortem pathology examination was declined by the family.
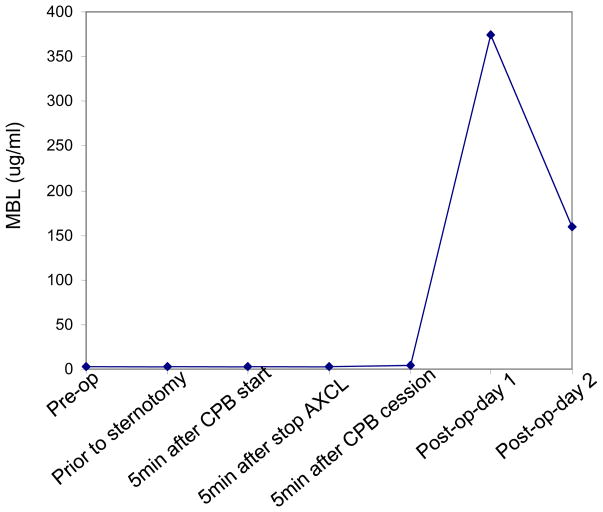
Prior to CABG, the patient was consented to have blood drawn at different time points for analysis of innate immune factors. Using specific immune assays, we found that the patient’s pre- and intra-operative blood samples had undetectable levels of MBL, but did have detectable levels of other complement factors, including C1q (the initial component in classical pathway), and Factor B (an early component in alternative pathway) (Table 2). Interestingly, the patient’s non-existent MBL levels increased to almost 400 ng/ml post-operatively (Figure 1).
Table 2.
ELISA results of initial factors of three complement pathways in the present case.
| Complement factor | Result | Normal value |
|---|---|---|
| MBL (lectin pathway) | < 1 ng/ml | ~1000 ng/ml |
| C1q (classical pathway) | 78 ug/ml | ~131 ug/ml |
| Factor B (alternative pathway) | 1.28 ug/ml | ~0.97 ug/ml |
Figure 1.
Plasma levels of MBL in the present case. Plasma samples were collected at different time points pre-, intra-, and post-operatively (as indicated on the x axis). MBL ELISA was performed using a Human MBL ELISA kit according to the instruction of manufacture (Hycult Biotechnology, Netherlands).
3. Comment
Our case is the first report of deficiency of MBL in a patient required cardiac surgery after an acute MI with an underlining CLL. The pre-existing condition of MBL deficiency may directly contribute to the pathogenesis of MI in this patient, as suggested by recent basic and clinical studies that link MBL to the development of cardiovascular diseases [2, 4–9]. One possible mechanism is that lack of MBL impairs the clearance of atherogenic factors, thus contributes to atherosclerosis and eventually MI in this patient.
Remarkably, this patient’s MBL level increased from undetectable pre- and intra-operatively to almost 400 ng/ml post-operatively day 1 (Figure 1). A possible source of this abrupt increase is fresh frozen plasma (FFP) transfusion during cardiac surgery (he received 3 units of FFP after the termination of CPB). A recent study found that FFP transfusion in patients with MBL deficiency was associated with MBL reconstitution and multiple organ dysfunction syndrome [10]. This may explain why our patient had an unfavorable outcome after cardiac surgery, possibly through transfused MBL which induced systemic inflammation. MBL mediated systemic inflammation in cardiac surgery was suggested by a study that MBL levels decrease at 30 and 240 minutes after termination of CPB compared with pre-operative levels, indicating activation of complement [11].
Major surgeries can cause stress response such as coagulopathy through consumption of clotting factors and fibrinolysis. Interestingly, a recent study showed open surgery for colorectal cancer induced a marked acute phase response, i.e. large increase in IL-6 and CRP levels with maximum at 12 h and 2 days. However, the levels of MBL were unaffected in these patients [12]. Thus, our study and others suggest that major surgery related MBL activation is more specific for heart.
There are several hypotheses for this patient’s pre-existing MBL deficiency. One is that it is a result of MBL genetic polymorphism. Three known structural mutations of MBL gene may attribute to the defective polymerization and thus low MBL level and dysfunction [13, 14]. MBL deficiency is estimated in about 5% of normal human population [13, 14], and approximately 30% of population has MBL levels below 500 ng/ml [1], (1000 ng/ml are considered sufficient for full activity of the MBL pathway). Significantly higher incidence of MBL deficiency-associated genotypes was found among patients with malignant disease compared [15]. Thus, this patient’s pre-existing MBL deficiency may contribute to the development of CLL.
Alternatively, this patient’s MBL deficiency may be a result of CLL progression that MBL produced in this patient were bound to malignant amount of B cells, thus sequestered from plasma and undetectable by immunoassay.
Further investigations of similar cases may elucidate the role of MBL in both malignancy and coronary artery disease, and may help clinical management of those patients with MBL deficiency and requiring cardiac surgery.
Acknowledgments
The authors would like to thank Dr. James Cottrell for his continued support, and Michael Lee, Amy Gleed, Hsiao-ying Chin, Donna Newman, Chris Johnson, and Downstate CT-ICU staffs for their valuable assistance.
References
- 1.PETERSEN SV, THIEL S, JENSENIUS JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–149. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- 2.COLLARD CD, VAKEVA A, MORRISSEY MA, et al. Complement activation after oxidative stress: role of the lectin complement pathway. Am J Pathol. 2000;156:1549–1556. doi: 10.1016/S0002-9440(10)65026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SAEVARSDOTTIR S, OSKARSSON OO, ASPELUND T, et al. Mannan binding lectin as an adjunct to risk assessment for myocardial infarction in individuals with enhanced risk. J Exp Med. 2005;201:117–125. doi: 10.1084/jem.20041431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FIANE AE, UELAND T, SIMONSEN S, et al. Low mannose-binding lectin and increased complement activation correlate to allograft vasculopathy, ischaemia, and rejection after human heart transplantation. Eur Heart J. 2005;26:1660–1665. doi: 10.1093/eurheartj/ehi198. [DOI] [PubMed] [Google Scholar]
- 5.MADSEN HO, VIDEM V, SVEJGAARD A, et al. Association of mannose-binding-lectin deficiency with severe atherosclerosis. Lancet. 1998;352:959–960. doi: 10.1016/S0140-6736(05)61513-9. [DOI] [PubMed] [Google Scholar]
- 6.BEST LG, DAVIDSON M, NORTH KE, et al. Prospective analysis of mannose-binding lectin genotypes and coronary artery disease in American Indians: the Strong Heart Study. Circulation. 2004;109:471–475. doi: 10.1161/01.CIR.0000109757.95461.10. [DOI] [PubMed] [Google Scholar]
- 7.OHLENSCHLAEGER T, GARRED P, MADSEN HO, JACOBSEN S. Mannose-binding lectin variant alleles and the risk of arterial thrombosis in systemic lupus erythematosus. N Engl J Med. 2004;351:260–267. doi: 10.1056/NEJMoa033122. [DOI] [PubMed] [Google Scholar]
- 8.LIMNELL V, AITTONIEMI J, VAARALA O, et al. Association of mannan-binding lectin deficiency with venous bypass graft occlusions in patients with coronary heart disease. Cardiology. 2002;98:123–126. doi: 10.1159/000066313. [DOI] [PubMed] [Google Scholar]
- 9.RUGONFALVI-KISS S, DOSA E, MADSEN HO, et al. High rate of early restenosis after carotid eversion endarterectomy in homozygous carriers of the normal mannose-binding lectin genotype. Stroke. 2005;36:944–948. doi: 10.1161/01.STR.0000160752.67422.18. [DOI] [PubMed] [Google Scholar]
- 10.BILGIN YM, BRAND A, BERGER SP, et al. Mannose-binding lectin is involved in multiple organ dysfunction syndrome after cardiac surgery: effects of blood transfusions. Transfusion. 2008;48:601–608. doi: 10.1111/j.1537-2995.2007.01585.x. [DOI] [PubMed] [Google Scholar]
- 11.MARCHEIX B, CARRIER M, MARTEL C, et al. Effect of pericardial blood processing on postoperative inflammation and the complement pathways. Ann Thorac Surg. 2008;85:530–535. doi: 10.1016/j.athoracsur.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 12.YTTING H, CHRISTENSEN IJ, BASSE L, et al. Influence of major surgery on the mannan-binding lectin pathway of innate immunity. Clin Exp Immunol. 2006;144:239–246. doi: 10.1111/j.1365-2249.2006.03068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.SORENSEN GL, PETERSEN I, THIEL S, et al. Genetic influences on mannan-binding lectin (MBL) and mannan-binding lectin associated serine protease-2 (MASP-2) activity. Genet Epidemiol. 2007;31:31–41. doi: 10.1002/gepi.20187. [DOI] [PubMed] [Google Scholar]
- 14.PRESANIS JS, KOJIMA M, SIM RB. Biochemistry and genetics of mannan-binding lectin (MBL) Biochem Soc Trans. 2003;31:748–752. doi: 10.1042/bst0310748. [DOI] [PubMed] [Google Scholar]
- 15.SWIERZKO AS, FLORCZAK K, CEDZYNSKI M, et al. Mannan-binding lectin (MBL) in women with tumours of the reproductive system. Cancer Immunol Immunother. 2007;56:959–971. doi: 10.1007/s00262-006-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]