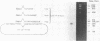Abstract
Oligodeoxyribonucleotides complementary to the DNA of the wild type (wt) bacteriophage phi chi 174 have been synthesized by the phosphotriester method. The oligomers, 11, 14, and 17 bases long, are complementary to the region of the DNA which accounts for the am-3 point mutation. When hybridized to am-3 DNA, the oligonucleotides form duplexes with a single base pair mismatch. The thermal stability of the duplexes formed between wt and am-3 DNAs has been measured. The am-3 DNA:oligomer duplexes dissociate at a temperature about 10 degrees C lower than the corresponding wt DNA:oligomer duplexes. This dramatic decrease in thermal stability due to a single mismatch makes it possible to eliminate the formation of the mismatched duplexes by the appropriate choice of hybridization temperature. These results are discussed with respect to the use of oligonucleotides as probes for the isolation of specific cloned DNA sequences.
Full text
PDF



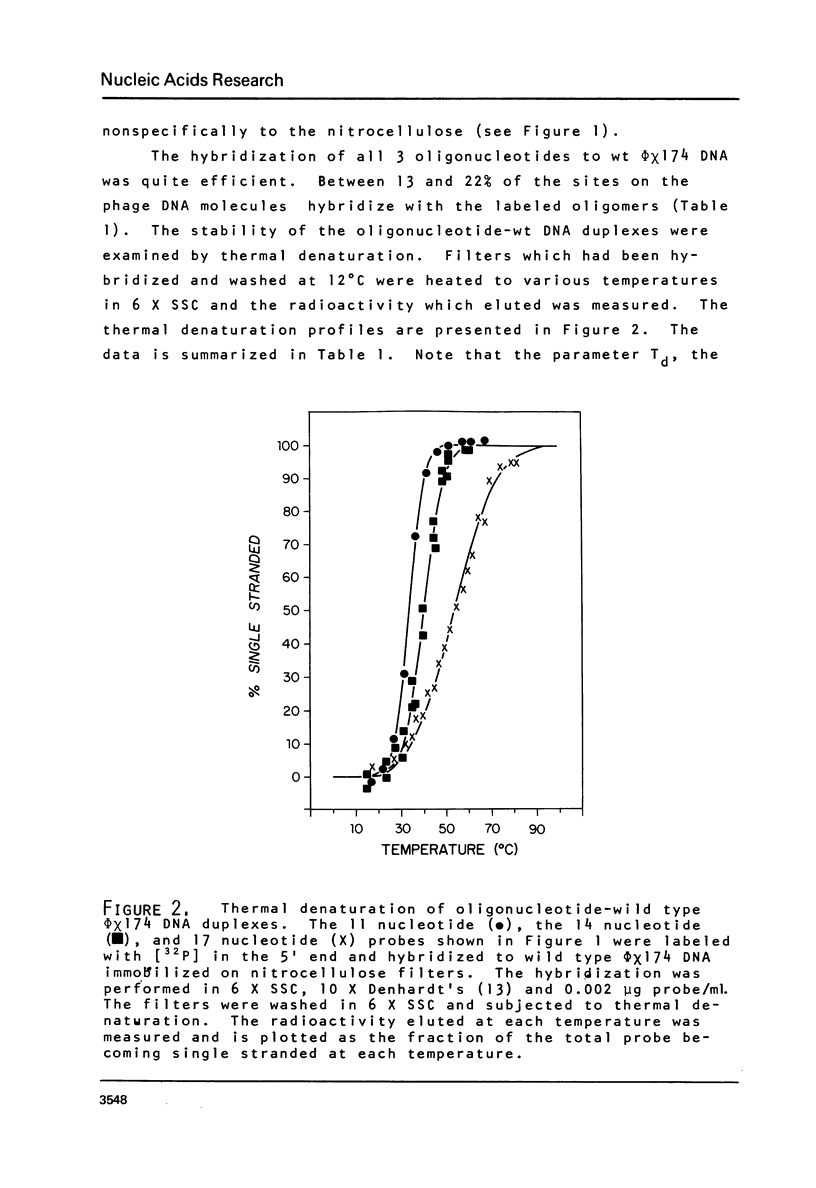
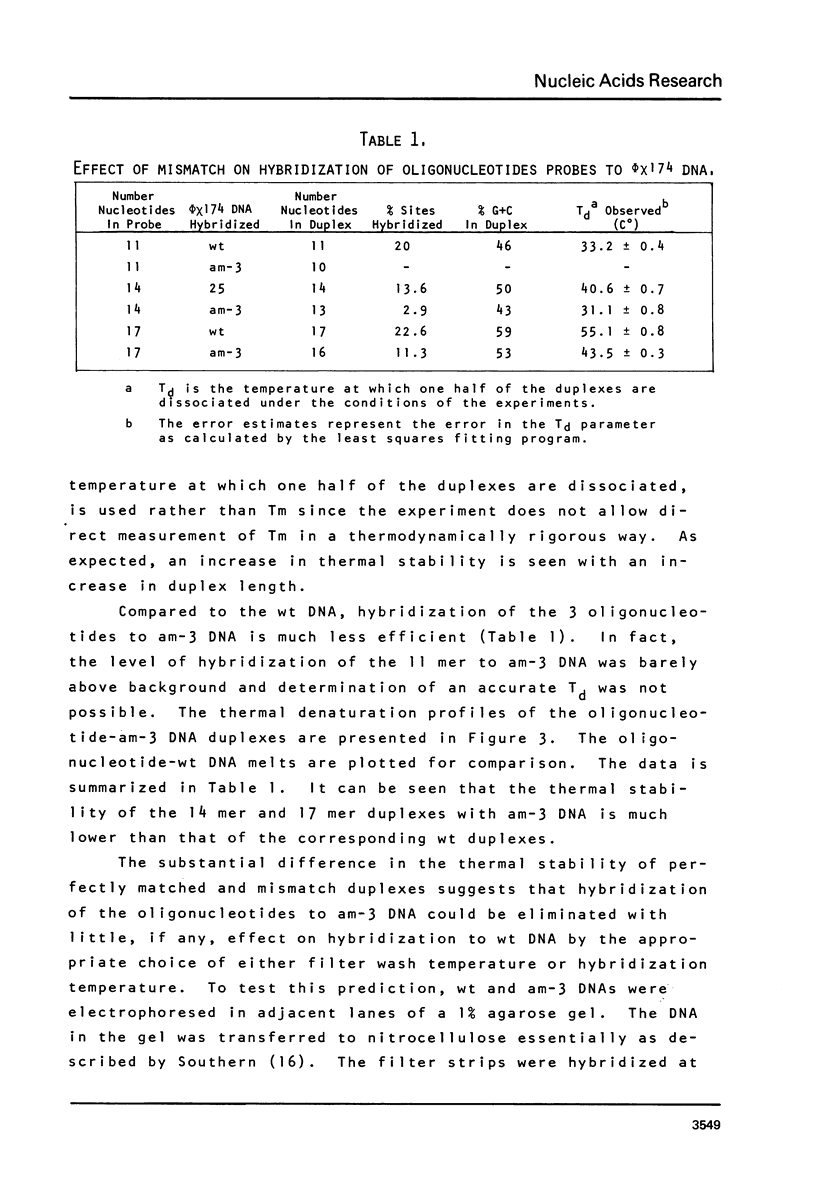
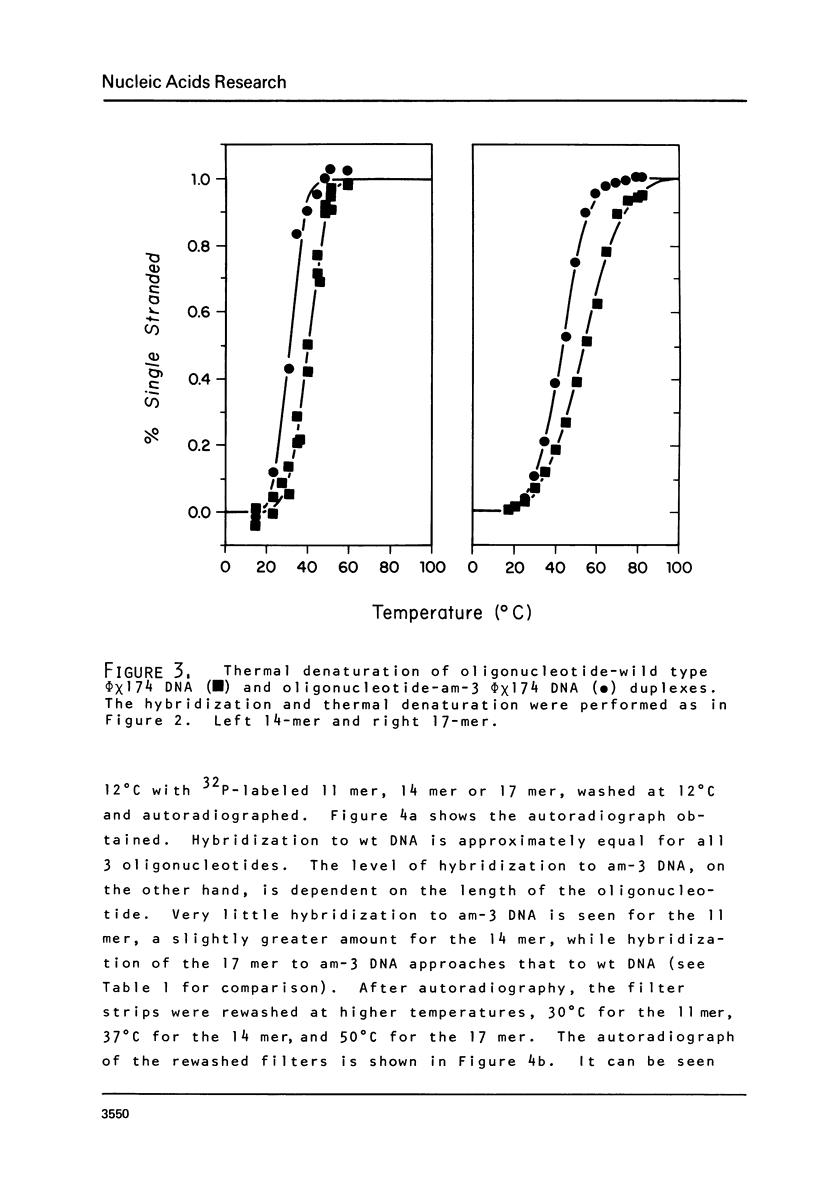
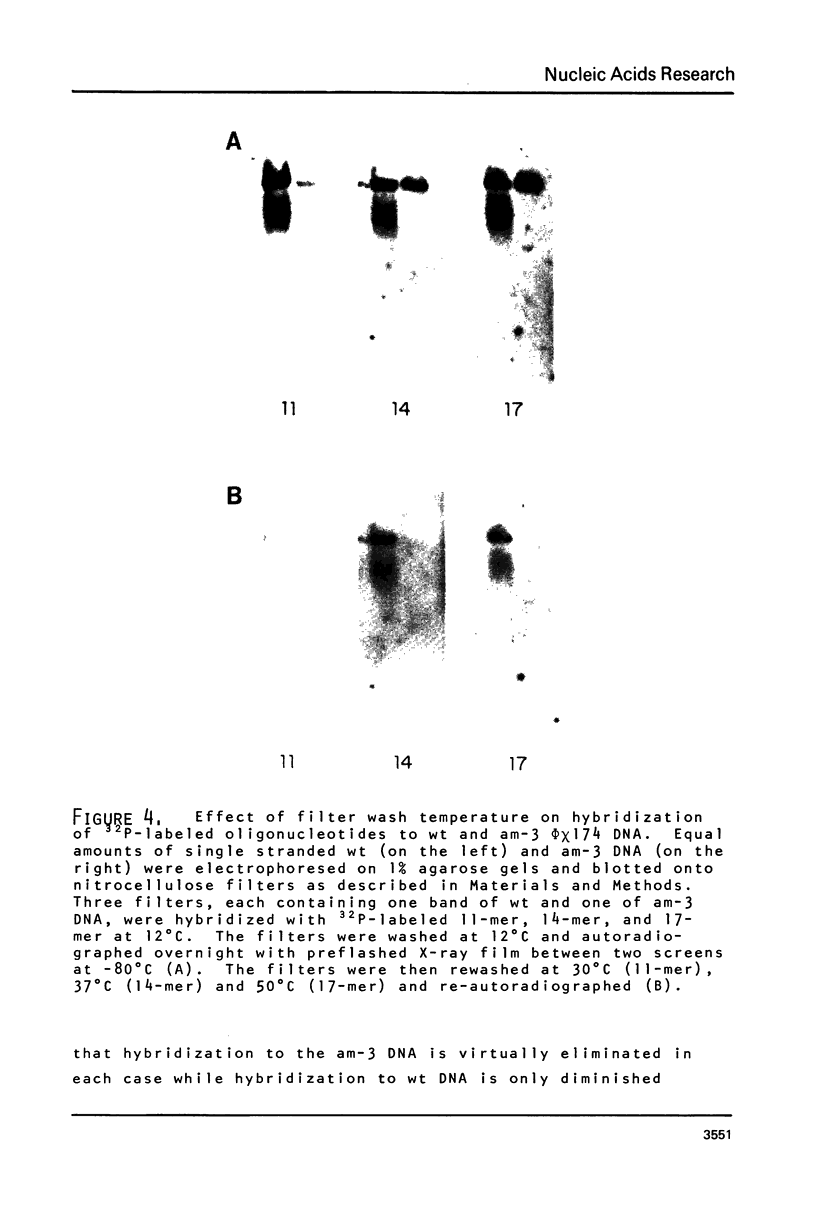





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Miller R. C., Jr, Caruthers M. H., Kumar A., Minamoto K., Van de Sande J. H., Sidarova N., Khorana H. G. Studies on polynucleotides. CXVII. Hybridization of polydeoxynucleotides with tyrosine transfer RNA sequences to the r-strand of phi80psu + 3 DNA. J Mol Biol. 1972 Dec 30;72(3):503–522. doi: 10.1016/0022-2836(72)90171-4. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Wells R. D. Synthesis and thermal melting behavior of oligomer-polymer complexes containing defined lengths of mismatched dA-dG and dG-dG nucleotides. Biochemistry. 1977 May 31;16(11):2367–2374. doi: 10.1021/bi00630a009. [DOI] [PubMed] [Google Scholar]
- Doel M. T., Smith M. The chemical synthesis of deoxyribo-oligonucleotides complementary to a portion of the lysozyme gene of phage T4 and their hybridization to phage specific RNA and phage DNA. FEBS Lett. 1973 Aug 1;34(1):99–102. doi: 10.1016/0014-5793(73)80712-4. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Lepennec J. P., Roskam W., Perrin F., Cami B., Krust A., Breathnach R., Chambon P., Kourilsky P. Isolation by molecular cloning of a fragment in the split ovalbumin gene. Nature. 1978 Jun 1;273(5661):349–354. doi: 10.1038/273349a0. [DOI] [PubMed] [Google Scholar]
- Gillam S., Waterman K., Smith M. The base-pairing specificity of cellulose-pdT9. Nucleic Acids Res. 1975 May;2(5):625–634. doi: 10.1093/nar/2.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Units of transcription and translation: sequence components of heterogeneous nuclear RNA and messenger RNA. Cell. 1975 Feb;4(2):77–93. doi: 10.1016/0092-8674(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Montgomery D. L., Hall B. D., Gillam S., Smith M. Identification and isolation of the yeast cytochrome c gene. Cell. 1978 Jul;14(3):673–680. doi: 10.1016/0092-8674(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Wu R. Nucleotide sequence analysis of DNA. IX. Use of oligonucleotides of defined sequence as primers in DNA sequence analysis. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1295–1302. doi: 10.1016/0006-291x(72)90852-2. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Hirose T., Itakura K., Riggs A. D. Efficient correction of a mutation by use of chemically synthesized DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4268–4270. doi: 10.1073/pnas.75.9.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Sedat J. W., Sinsheimer R. L. Structure of the DNA of bacteriophage phiX174. VII. Methylation. J Mol Biol. 1970 Oct 28;53(2):251–259. doi: 10.1016/0022-2836(70)90298-6. [DOI] [PubMed] [Google Scholar]
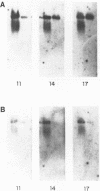
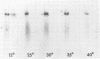
- Sanger F., Air G. M., Barrell B. G., Brown N. L., Coulson A. R., Fiddes C. A., Hutchison C. A., Slocombe P. M., Smith M. Nucleotide sequence of bacteriophage phi X174 DNA. Nature. 1977 Feb 24;265(5596):687–695. doi: 10.1038/265687a0. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Khorana H. G. CXII. Total synthesis of the structural gene for an alanine transfer RNA from yeast. Enzymic joining of the chemically synthesized polydeoxynucleotides to form the DNA duplex representing nucleotide sequence 1 to 20. J Mol Biol. 1972 Dec 28;72(2):427–444. doi: 10.1016/0022-2836(72)90155-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Brack C., Hozumi N., Schuller R. Cloning of an immunoglobulin variable region gene from mouse embryo. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3518–3522. doi: 10.1073/pnas.74.8.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C., Martin F. H., Doty P. Self-complementary oligoribonucleotides: effects of helix defects and guanylic acid-cytidylic acid base pairs. J Mol Biol. 1971 Apr 28;57(2):217–229. doi: 10.1016/0022-2836(71)90342-1. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Wu R. Nucleotide sequence analysis of DNA. Nat New Biol. 1972 Apr 19;236(68):198–200. doi: 10.1038/newbio236198a0. [DOI] [PubMed] [Google Scholar]