Figure 3. Comparison of the α-NRXN_2-6 LNS domains.
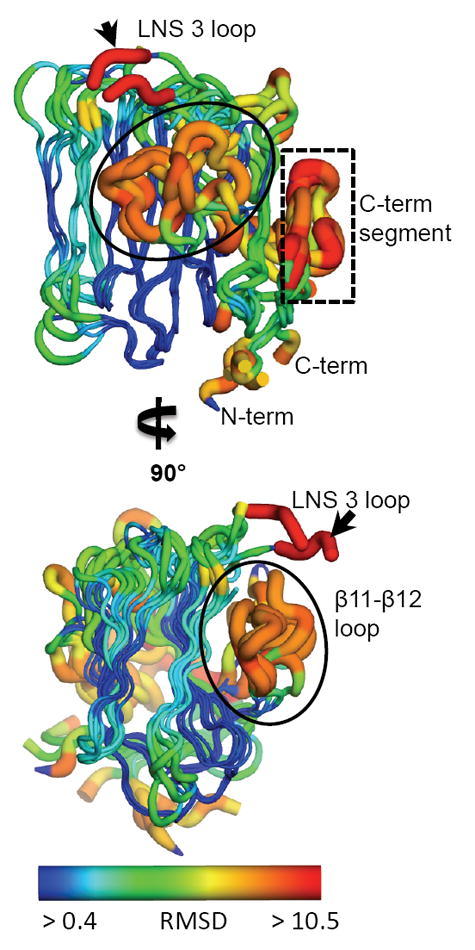
The five LNS domains in the α-NRXN_2-6 structure share a conserved conformation with a characteristic 13 β-sheet fold. Regions of high variation, measured by deviation in the Cα backbone, include the β11-β12 loop (circled), a loop at the Ca2+-binding face in LNS 3 (small black arrow), and the C-terminal segment including the α-helix and proceeding β-sheet (boxed). Relative variation is depicted by color and size of the tube, where thin blue tubes represent low variation and wide orange tubes show greater variation. Residues that do not align with any other residues in a sequence alignment of all five domains are colored in red. (also see Supplemental Figure S2).
