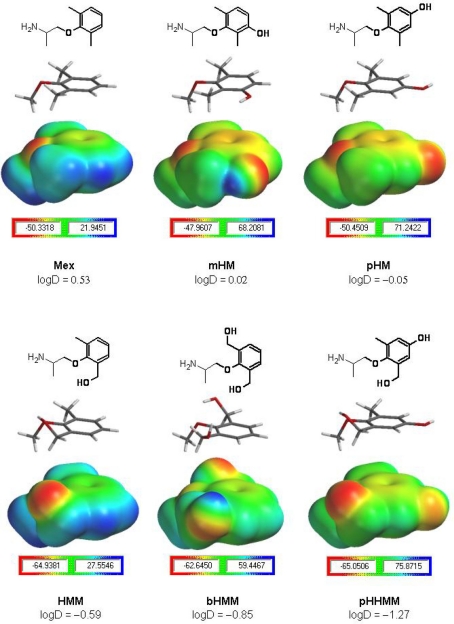
Figure 8.
Calculation of drug electrostatic potential. Mexiletine and its hydroxylated analogs are displayed as chemical structures presenting in bold the moiety studied by ab initio calculations (upper panels). For each compound, both the corresponding HF/3-21G* minimized three-dimensional structure and the electrostatic potential map is given in equatorial and lower panels, respectively. For each moiety, the scale bar indicates the electrostatic potential surface value range in kilocalorie per mole, the negative value being the one used in structure–activity evaluations. The HMM, bHMM, and pHHMM moieties (lower half of the figure) presented the most negative electrostatic potential values (around −65 kcal/mol), which most likely may allow less favorable interactions with electron-rich aromatic rings (e.g., F1586 or Y1593 rings), compared to the ones expected for Mex, mHM, and pHM moieties with a less negative electrostatic potential (around −50 kcal/mol). The Log D values experimentally measured at pH 7.4 are indicated.