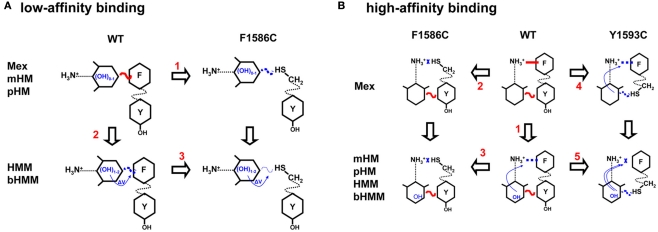
Figure 9.
Synopsis of possible interactions of mexiletine and hydroxylated analogs with the low (A) and high (B) binding site within the wild-type hNav1.4 channel and its mutants F1586C and Y1593C. (A) Regarding the low-affinity binding to closed channels, our results are in accord with an horizontal orientation of the drug, with the aryloxy moiety interacting either directly or indirectly with F1586 through electrostatic coupling. The addition of OH groups as in mHM and pHM does not modify aryloxy electrostatic potential thereby leaving this interaction unaltered. The F1586C mutation weakens interaction with Mex, mHM, and pHM to the same extent (arrow 1). Introduction of OH as in HMM and bHMM weakens the interaction due to modification of aryloxy electrostatic potential and possibly other parameters (arrow 2). The F1586C mutation produces additional weakening of the interaction with HMM and bHMM (arrow 3). Finally the Y1593C mutation has no effect on low-affinity binding (not illustrated). (B) Regarding the high-affinity binding to inactivated channels, addition of OH groups to the aryloxy moiety cooperatively reduced the interaction of charged amine group with F1586 (arrow 1). The amplitude of this effect depends on the OH position and lipophilicity. The F1586C mutation disrupts the π–cation interaction, so that OH substitutions have no more influence on binding (arrow 2 and 3). In contrast, the Y1593C mutation impairs only partially the π–cation interaction of mexiletine through cooperativity (arrow 4). The mutation also further impairs the binding of hydroxylated analogs (arrow 5).