Table 1.
Chemical structures and physicochemical properties.
| Compound | Structure | Log P | pKa | Log D (pH 7.4) | Ionization (mol%, pH 7.4) | Electrostatic potential (kcal/mol) |
|---|---|---|---|---|---|---|
| Mexiletine | 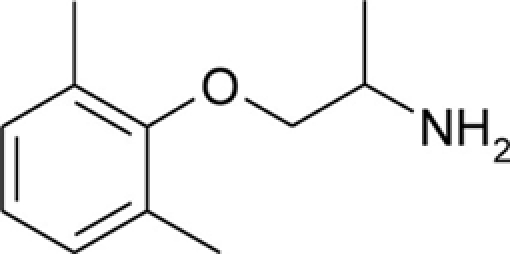 |
2.21 ± 0.01 | 9.28 ± 0.01 | 0.53 | 98.7 | −50.3 |
| mHM | 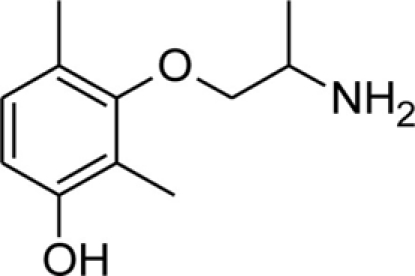 |
1.67 ± 0.01 | 9.04 ± 0.01 | 0.02 | 97.7 | −48.0 |
| pHM | 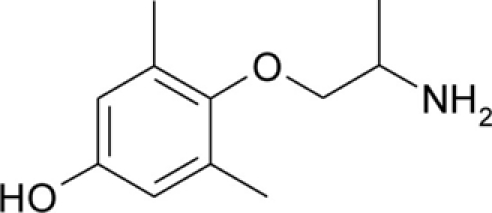 |
1.53 ± 0.01 | 8.97 ± 0.01 | −0.05 | 97.3 | −50.4 |
| HMM | 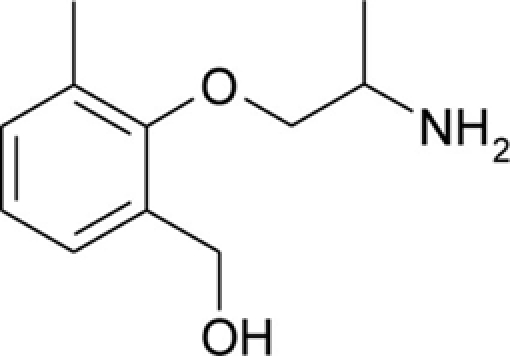 |
1.15 ± 0.01 | 9.13 ± 0.01 | −0.59 | 98.1 | −64.9 |
| bHMM | 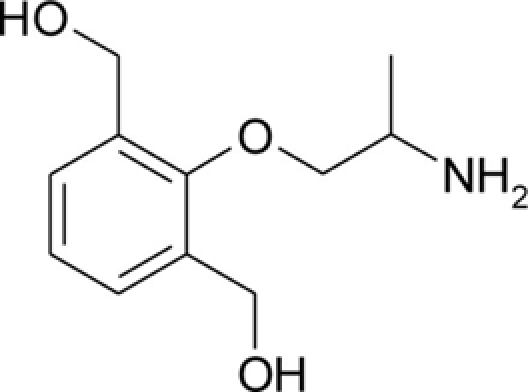 |
0.25 ± 0.05 | 9.21 ± 0.03 | −0.85 | 98.5 | −62.6 |
| pHHMM | 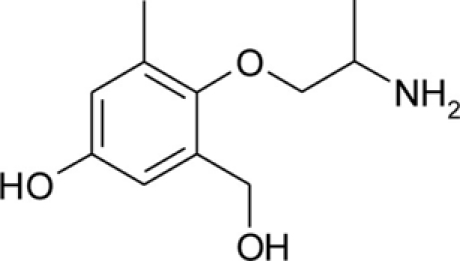 |
0.23 ± 0.02 | 8.89 ± 0.02 | −1.27 | 96.8 | −65.0 |
The Log P, pKa, Log D, and electrostatic potential values were determined experimentally, as described in the Section “Materials and Methods.” Values of Log P and pKa are given as mean ± SEM. Ionization of the amine group at pH 7.4 was calculated from Henderson–Hasselbalch equation,
