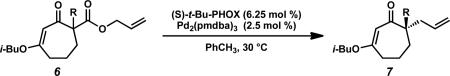
Table 2.
Scope of the Pd-catalyzed enantioselective alkylation of cyclic vinylogous esters.a
 | |||||
|---|---|---|---|---|---|
| entry | substrate 6 | R | product 7 | yieldb(%) | eec(%) |
| 1 | 6a | –CH3 | 7a | 91 | 88 |
| 2 | 6b | –CH2CH3 | 7b | 89 | 92 |
| 3 | 6c | –CH2Ph | 7c | 98 | 86 |
| 4 | 6d | –CH2C≡CH | 7d | 88 | 89 |
| 5 | 6e | –CH2CH2CH=CH2 | 7e | 95 | 87 |
| 6 | 6f |
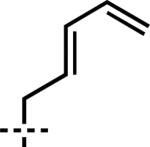
|
7f | 90 | 90 |
| 7 | 6g |
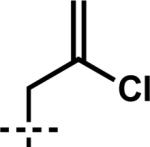
|
7g | 99 | 86 |
| 8 | 6h | –CH2CH2CN | 7h | 96 | 87 |
| 9 | 6i |
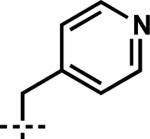
|
7i | 97 | 85 |
| 10 | 6j |
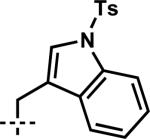
|
7j | 98 | 83 |
| 11 | 6k |
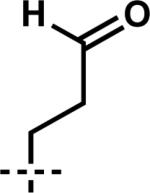
|
7k | 90 | 80 |
| 12 | 61 | –F | 71 | 94 | 91 |
| 13 | 6m | –CH2OTBDPSd | 7m | 66 | 58 |
| 14 | 6n |
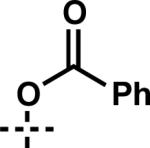
|
7n | 75 | 57 |
Conditions: β-ketoester 6 (1.0 equiv), Pd2(pmdba)3 (2.5 mol %), (S)-t-Bu-PHOX (L1, 6.25 mol %) in PhCH3 (0.1 M) at 30 °C; pmdba = 4,4’-methoxydibenzylideneacetone.
Isolated yield.
Determined by chiral HPLC or SFC.
TBDPS = tert-butyldiphenylsilyl, Ts = 4-toluenesulfonyl.
