Table 3.
Ring contraction reaction optimization.a
 | |||||
|---|---|---|---|---|---|
| entry | base | additive | solvent | T (°C) | yieldb(%) |
| 1 | LiOt-Bu | — | t-BuOH | 40 | 71 |
| 2 | LiOt-Bu | — | THF | 40 | 60 |
| 3 | NaOt-Bu | — | THF | 40 | 81 |
| 4e | KOt-Bu | — | THF | 40 | 85 |
| 5 | NaOH | — | THF | 60 | 89 |
| 6 | KOH | — | THF | 60 | 87 |
| 7 | LiOH | — | THF | 60 | 19d |
| 8 | LiOH | t-BuOH | THF | 60 | 78 |
| 9 | LiOH | HFIPc | THF | 60 | 87 |
| 10 | LiOH | TFEc | THF | 60 | 96 |
| 11 | LiOCH2CF3 | — | THF | 60 | 90e |
| 12 | CsOH·H2O | — | THF | 60 | 48 |
| 13 | Cs2CO3 | — | THF | 60 | 61f |
| 14 | Cs2CO3 | TFEc | THF | 60 | 86 |
| 15 | Cs2CO3 | TFEc | CH3CN | 60 | 100 |
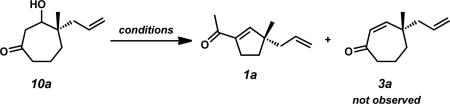
Conditions: β-hydroxyketone 10a (1.0 equiv), additive (1.5 equiv), base (1.5 equiv), solvent (0.1 M) at indicated temperature for 9–24 h.
GC yield using an internal standard at ≥ 98% conversion unless otherwise stated.
HFIP = 1,1,1,3,3,3-hexafluoro-2-propanol; TFE = 2,2,2-trifluoroethanol.
Several reaction intermediates observed by TLC and GC analysis; proceeded to 78% conversion.
Isolated yield.
Reaction did not reach completion at 24 h; proceeded to 67% conversion.
