Table 4.
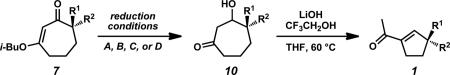 | ||||||
|---|---|---|---|---|---|---|
| entry | substrate 7 | reduction conditions | R1 | R2 | product 1e | yield (%)f |
| 1 | 7a | A | –CH3 | –CH2CH=CH2 | 1a | 84 |
| 2 | 7b | A | –CH2CH3 | –CH2CH=CH2 | 1b | 90 |
| 3 | 7c | A | –CH2Ph | –CH2CH=CH2 | 1c | 86 |
| 4 | 7d | A | –CH2C≡CH | –CH2CH=CH2 | 1d | 95 |
| 5 | 7e | A | –CH2CH2CH=CH2 | –CH2CH=CH2 | 1e | 87 |
| 6 | 7f | A |
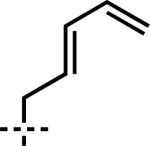
|
–CH2CH=CH2 | 1f | 91 |
| 7 | 7g | A |
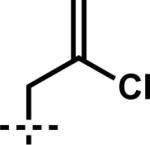
|
–CH2CH=CH2 | 92 | |
| 8 | 7h | A | –CH2CH2CN | –CH2CH=CH2 | 1h | 85 |
| 9 | 7i | B |
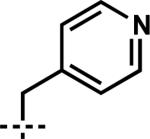
|
–CH2CH=CH2 | 1i | 80 |
| 10 | 7j | A |
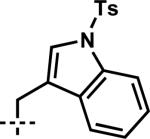
|
–CH2CH=CH2 | 1j | 87 |
| 11 | 7m | C | –CH2OTBDPSi | –CH2CH=CH2 | 1m | 91 |
| 12 | 7o g | C | –(CH2)3OTBDPSi | –CH2CH=CH2 | 1o | 85 |
| 13 |
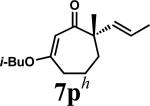
|
A |
|
1p | 81 | |
| 14 |
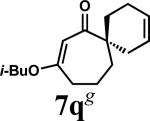
|
A |
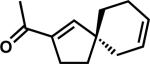
|
1q | 87 | |
| 15 | 7n | D | –OH | –CH2CH=CH2 | 1n | 25 |
| 16 | 7l | A | –F | –CH2CH=CH2 | 1l | 0 |
Reduction Conditions A: vinylogous ester 7 (1.0 equiv), LiAlH4 (0.55 equiv) in Et2O (0.2 M) at 0 °C, then 10% aqueous HCl quench.
Reduction Conditions B: 1) vinylogous ester 7 (1.0 equiv), DIBAL (1.2 equiv) in PhCH3 (0.03 M) at –78 °C; 2) oxalic acid·2H2O in MeOH (0.02 M).
Reduction Conditions C: vinylogous ester 7 (1.0 equiv), CeCl3·7H2O (1.0 equiv), NaBH4 (3.0 equiv) in MeOH (0.02 M) at 0 °C, then 10% aqueous HCl in Et2O at 0 °C.
Reduction Conditions D: vinylogous ester 7 (1.0 equiv), DIBAL (3.3 equiv) in PhCH3 (0.03 M) at –78 °C; 2) 10% aqueous HCl in Et2O at 0 °C.
Ring Contraction Conditions: β-hydroxyketone 10 (1.0 equiv), CF3CH2OH (1.5 equiv), LiOH (1.5 equiv) in THF (0.1 M) at 60 °C.
Isolated yield over 2-3 steps.
Prepared from 7k.
h Prepared from 7a.
TBDPS = tert-butyldiphenylsilyl, Ts = 4-toluenesulfonyl, DIBAL = diisobutylaluminum hydride.
