Summary
Background and objectives
CKD is a risk factor for medial artery calcification, but the CKD stage at which this risk begins is unknown. Because breast arterial calcification (BAC) is a marker of generalized medial arterial calcification, mammography was used to detect medial arterial calcification in women with different CKD stages.
Design, setting, participants, & measurements
This was a retrospective, cross-sectional study of women with and without CKD matched for age and diabetes and identified from mammograms obtained in 2006–2011. BAC was scored as present or absent per visual inspection.
Results
A total of 146 women with stage 3 CKD and 54 with stage 4/5 CKD were identified. An additional 21 patients with ESRD were identified and added to a previous cohort of 71 patients. Mean age was 64 years for CKD 3, 63 for CKD 4, and 59 for ESRD. Half of each group had diabetes. Compared with controls, the odds ratios for BAC were 1.44 in CKD 3 (95% confidence interval [CI], 0.82–2.53), 2.69 in CKD 4 (95% CI, 1.14–6.33), and 7.19 in ESRD (95% CI, 3.77–13.7) and did not differ with diabetic status or race. In a multivariable logistic model, age (P<0.001) and estimated GFR (P=0.005) were independent predictors of BAC. The odds ratio for BAC increased 4% for each milliliter per minute per 1.73 m2 decrease in estimated GFR. The prevalence of BAC in CKD was increased in each decade of age over 49 years.
Conclusions
CKD is an independent risk factor for medial arterial calcification.
Introduction
The risk for cardiovascular disease is very high in patients with advanced CKD but cannot be completely explained by traditional risk factors for atherosclerosis, suggesting that a different disorder is responsible. Another vascular lesion that occurs in CKD and ESRD and could contribute to clinical cardiovascular disease is calcification of the medial (smooth muscle) layer of arteries, also known as Mönckeberg arteriosclerosis. Medial arterial calcification (MAC) is distinct from the neointimal calcification of atherosclerosis (1) and can occur in the absence of atherosclerosis. Whereas atherosclerosis underlies ischemic heart disease, MAC decreases arterial compliance, potentially leading to left ventricular hypertrophy and congestive heart failure (2). In addition to ESRD and CKD, MAC also occurs with advanced age and diabetes. The extent to which aging and diabetes contribute to the prevalence of medial calcification in CKD or to which CKD explains the prevalence of medial calcification in aging and diabetes is also unknown. Combined effects of aging and CKD on medial calcification could help explain the high rates of cardiovascular disease and mortality in this population.
Information on medial calcification in humans is very limited because imaging techniques currently used cannot reliably distinguish it from intimal calcification associated with atherosclerosis. Computed tomography of the coronary arteries or aorta is frequently used and has shown increased calcification in CKD (3–5), but whether this represents medial or atherosclerotic calcification, or both, is unclear. Thus, the true prevalence of medial calcification in CKD and when in the course of CKD the risk for MAC begins to increase are unknown. This information is critical for targeting preventive measures. Arterial calcification is easily identified by mammography, and we have shown that this calcification is exclusively medial and correlates well with calcification in other peripheral arterial beds (6). Because mammography is routinely performed, breast arterial calcification (BAC) provides a convenient window into medial calcification in humans without necessitating additional radiation exposure. We therefore used screening mammography to determine for the first time the prevalence of medial calcification and its association with diabetes at different stages of CKD.
Materials and Methods
Patients
Patients were randomly selected from a computerized search of all Emory Healthcare patients who underwent screening mammography between 2006 and 2011 and had been assigned a diagnosis code for CKD or had an elevated serum creatinine concentration. The diagnosis of CKD was based on a serum creatinine value >1.0 mg/dl measured within 6 months before or after the mammogram. If additional measurements were available within that time frame, they were averaged; this occurred in approximately 80% of the patients. Values determined in the inpatient setting were excluded. CKD stage was based on estimated GFR (eGFR) in ml/min per 1.73 m2, as determined by the four-variable Modification of Diet in Renal Disease formula (7) and was categorized as stage 3 (30 ml/min per 1.73 m2 < eGFR < 60 ml/min per 1.73 m2) and stage 4/5 (eGFR < 30 ml/min per 1.73 m2 without renal replacement therapy). Patients 80 years of age and older, patients with kidney transplantation or liver disease, or those lacking suitable creatinine values were excluded. ESRD was defined as long-term hemodialysis or peritoneal dialysis at the time of mammography. Patients with ESRD were added to a previously published cohort (6) and were used solely to define the upper range for BAC prevalence; they were not used in any other analyses. For each patient, a control participant without CKD was randomly selected from among all women undergoing screening mammography between 2006 and 2011 and was matched for age (±1 year), diabetes, and, when possible, race. All patients were selected without knowledge of the mammogram result.
Absence of CKD was defined as a serum creatinine value <1.0 mg/dl obtained within 6 months before or after the mammogram. Data on mineral metabolism were not collected because they were not available in many patients and the relationship of the samples to meals could not be determined. Diabetes was defined as a history of diabetes or use of insulin or oral hypoglycemic agents as determined by review of the medical records. Cardiovascular disease was defined as a history of coronary artery disease (myocardial infarction, coronary artery bypass graft surgery, coronary stent, or presence of stenosis on coronary angiography), heart failure (ejection fraction < 45% or the presence of moderate or severe diastolic dysfunction by echocardiography), or peripheral arterial disease (claudication, evidence of vascular disease on imaging, or vascular bypass surgery). Race was also obtained from the medical records.
Mammograms
Mammograms were retrieved in digital or film format. Screening of mammograms for BAC was done by visual inspection by a single reader and was restricted to determination of whether BAC was present or absent. A second reader scored 30% of the mammograms, and the agreement between those scores was 97%.
Statistical Analyses
Univariate analysis was performed using a t test for continuous variables and a chi-squared test for categorical variables. Multivariate logistic regression analysis was performed with SPSS 17.0 software (SPSS, Inc., Chicago, IL).
Results
A total of 292 patients were considered for analysis and compared with an equal number of controls (Table 1). Of these, 146 women had CKD stage 3, 54 had CKD stage 4/5, and 92 had ESRD and were undergoing long-term dialysis. Of the CKD 4/5 group, 51 had CKD 4 and 3 had CKD 5 (not receiving dialysis). The ESRD group consisted of a previously reported cohort of 71 patients with ESRD (6) and an additional 21 women who were identified during the current study. The patients with CKD 3 and CKD 4/5 had similar ages, but those with ESRD were slightly younger. Approximately half of the patients had diabetes, with equal proportions in all groups. Almost all patients with ESRD and two thirds of patients in both CKD groups were African American. Cardiovascular disease was present in 35% of the 191 patients with CKD for whom these data were obtained.
Table 1.
Characteristics of patients and controls
| Characteristic | CKD 3 | CKD 4/5 | ESRD | |||
|---|---|---|---|---|---|---|
| Patients | Controls | Patients | Patients | Patients | Controls | |
| Participants (n) | 146 | 146 | 77 | 77 | 92 | 92 |
| Creatinine (mg/dl) | 1.53±0.25 | 0.80±0.10 | 2.86±0.75 | 0.80±0.11 | 0.80±0.11 | |
| Age (yr) | 64.1±10.2 | 64.2±10.3 | 63.6±10.0 | 64.5±11.5 | 59.2±12.0 | 59.4±12.0 |
| African American (%) | 72 | 55 | 74 | 67 | 98 | 68 |
| Diabetes (%) | 57 | 57 | 62 | 67 | 58 | 58 |
Data expressed with a plus/minus sign are mean ± SD.
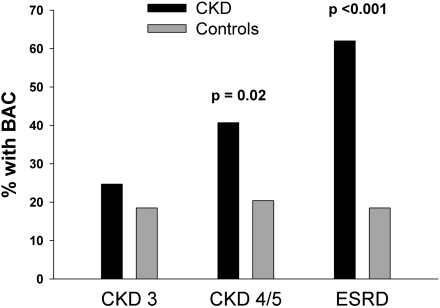
The prevalence of BAC (Figure 1) was increased above the prevalence in controls in each group, although this difference was not significant for CKD 3. This yielded odds ratios of 1.44 (95% confidence interval [CI], 0.82–2.53) for CKD 3, 2.69 (95% CI, 1.14–6.33) for CKD 4/5, and 7.19 (95% CI, 3.77–13.7) for ESRD. For all patients with CKD combined, the prevalence of BAC was 29.0%, compared with 19.0% in the controls, yielding an odds ratio of 1.7 (95% CI, 1.09–2.70). The prevalence did not vary between control groups. Patients with diabetes did not have a greater prevalence of BAC than patients without diabetes, and prevalence did not vary by race (Table 2).
Figure 1.
Prevalence of breast arterial calcification (BAC) in women with CKD and in women without CKD matched for age and diabetes.
Table 2.
Prevalence of breast arterial calcification by diabetic status and race
| Variable | Breast Arterial Calcification (%) | ||
|---|---|---|---|
| Patients with CKD | Controls | ||
| Diabetes | |||
| yes | 26.3 | 19.3 | |
| no | 32.6 | 18.6 | |
| African American | |||
| yes | 28.8 | 17.9 | |
| no | 29.6 | 20.5 | |
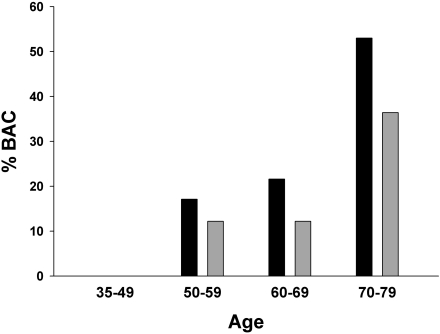
In a multivariable logistic regression that included eGFR, age, diabetes, and race, both age and eGFR were significant predictors of BAC in patients with CKD (Table 3). The odds ratio for BAC increased approximately 4% for each ml/min per 1.73 m2 decrease in eGFR and 13% per year of age. The association between age and BAC was similar in the control group. Estimated GFR was not a determinant of BAC in the logistic regression for the control group. Because age is a determinant of eGFR, the patients were stratified by age to confirm the association of CKD with BAC independent of age (Figure 2). The prevalence of BAC was consistently higher in patients with CKD than in the controls in all age groups, with odds ratios of 1.48 (95% CI, 0.43–5.12), 1.99 (95% CI, 0.82–4.80), and 1.97 (95% CI, 0.98–3.97) with increasing decades. The association of CKD with BAC could not be determined in women younger than age 50 because of the absence of BAC in this small sample.
Table 3.
Multivariable logistic regression for breast arterial calcification
| Variable | Patients with CKDa | Controls | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.13 (1.08–1.18) | <0.001 | 1.12 (1.06–1.18) | <0.001 |
| eGFR | 0.96 (0.92–0.99) | 0.005 | 1.00 (0.97–1.03) | 0.96 |
| African American | 1.38 (0.62–3.1) | 0.43 | 0.96 (0.44–2.12) | 0.92 |
| Diabetes | 0.65 (0.32–1.32) | 0.23 | 1.11 (0.50–2.47) | 0.80 |
OR, odds ratio; CI, confidence interval; eGFR, estimated GFR.
Stages 3, 4, and 5 not undergoing dialysis.
Figure 2.
Prevalence of breast arterial calcification (BAC) at different ages in women with CKD and in women without CKD matched for age and diabetes. Calcification was not present in any of the 19 patients or controls younger than age 50 years.
Discussion
The prevalence of MAC is believed to be increased in CKD and ESRD, but the precise risk, its onset, and its association with other variables (such as age and diabetes) remain unknown because of the lack of specificity of imaging modalities used. This study, using BAC as a marker of MAC, demonstrates that the risk may begin in stage 3 CKD and is clearly present by stage 4. This finding suggests that preventive or therapeutic strategies may need to be implemented as early as stage 3 CKD. Although these finding are limited to intramammary arteries, we have previously shown that this calcification correlates with peripheral arterial calcification in the extremities (6). Thus, it is likely that these results indicate generalized MAC.
Although mineral metabolism was not studied, the results can be compared with the known alterations in mineral metabolism at different stages of CKD (8). Whereas levels of parathyroid hormone and fibroblast growth factor 23 are elevated in stage 3 CKD, the prevalence of hyperphosphatemia is still low but increases sharply in stage 4 CKD, corresponding to the significant increase in BAC. This implicates hyperphosphatemia as opposed to the other abnormalities in the onset of MAC, a result consistent with a large body of experimental data indicating the pathogenic role of phosphate (9,10). However, this does not rule out a role for parathyroid hormone or fibroblast growth factor 23; confirmation of this possibility and whether the smaller increase in risk for MAC in stage 3 CKD is related to hyperphosphatemia will require future prospective studies directly correlating MAC with mineral metabolism.
The prevalence of MAC is known to increase with age, and this has been demonstrated in numerous studies of BAC (11–16). Although the prevalence of CKD increases with age and age is used to calculate eGFR and therefore to assign CKD stage, this cannot explain the increased prevalence of BAC in CKD. Patients were matched for age, and CKD increased the prevalence of BAC at all ages, indicating that the risk for MAC in CKD is not explained by age. Furthermore, the similar odds ratios for BAC with age in the CKD and control groups in the logistic regression argue against any significant interaction between age and CKD. Although the age-related increase in medial calcification was proportionately similar in patients with CKD and controls, a combined effect of age and CKD on medial calcification may contribute to the high prevalence of cardiovascular disease in older patients with CKD. The prevalence of BAC did not differ between African Americans and patients of other races (consistent with findings in the general population [14]), but our sample size limited the power to detect a true difference based on race. Thus, the results do not necessarily apply to all populations of patients with CKD.
Medial calcification has been related to diabetes in previous studies of peripheral arteries (17–20), but the data with breast arteries are less conclusive: Some studies have shown a link with diabetes (14–16,21,22) and others show no link (13,23–27), as was seen in the present study. None of the previous studies accounted for CKD, which is common in patients with diabetes and could explain an association with MAC. In addition, most studies did not consider the duration of diabetes, which appears to correlate strongly with MAC (18–20). MAC is particularly common in patients with diabetes and severe neuropathy (20,28), again consistent with duration. However, duration and the presence of other diabetic complications also correlate with diabetic nephropathy; this finding also suggests that CKD could contribute to the risk for MAC in diabetes.
This study has several limitations, including its retrospective, cross-sectional nature; the lack of data on mineral metabolism and treatment; the lack of quantification of BAC; and the fact that BAC is not a fully validated marker of generalized MAC. Although BAC does correlate with apparent medial calcification in peripheral arteries, the correlation with medial calcification in larger arteries remains untested. Because of the sample size, the increase in BAC prevalence in CKD 3 did not reach statistical significance. This is due in part to the low prevalence of CKD in women undergoing screening mammography, which, in turn, may be related to low mammography rates in populations with a high prevalence of CKD. Because the correlation of eGFR with true GFR is poor in individuals with a normal serum creatinine level, there is the potential for misclassification in this study. In particular, it is possible that some of the older controls had CKD and that the relative risk for BAC in CKD is actually greater than indicated by the data. However, the fact that eGFR did not correlate with BAC in the controls argues against this. Single creatinine values can also lead to misclassification, but multiple values were available in 80% of the patients with CKD.
Other risk factors that may be associated with increased risk for BAC, including parity and hormonal therapy (14,21,22), could not be reliably ascertained and were not included. However, we are unaware of any linkage between these variables and CKD. Additional risk factors for vascular disease, such as hypertension and smoking, were also not included. Because hypertension is present in more than 80% of people with CKD (29,30), its contribution to BAC would be difficult to detect. A study of almost 13,000 women did not find an independent association of hypertension with BAC (14), indicating that hypertension is unlikely to explain the association of CKD with medial calcification. Information on smoking can be difficult to obtain from chart review, particularly in terms of duration and timing. Although smoking is associated with CKD (31), it imparts a decreased risk for BAC in the general population (14,27). Thus, it should not account for CKD as a risk factor for BAC but could obscure its relation with CKD.
In summary, our data demonstrate that the risk for BAC, used as a surrogate for medial calcification, may begin in stage 3 CKD and become significantly higher in stage 4. Although diabetes and race were not associated with increased risk for BAC in this study, age and eGFR were major determinants of this risk. These results suggest that preventive strategies may need to begin as early as stage 3 CKD. Additional studies are needed to determine whether the risk for MAC in CKD is explained by specific abnormalities in mineral metabolism.
Acknowledgments
This work was supported by National Institutes of Health Grant DK068691 and a grant from Genzyme Corporation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Amann K: Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol 3: 1599–1605, 2008 [DOI] [PubMed] [Google Scholar]
- 2.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Adler S: Coronary artery calcification in nondialyzed patients with chronic kidney diseases. Am J Kidney Dis 45: 963, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra R, Budoff M, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler S: Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int 66: 2022–2031, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC: Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis 27: 394–401, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Duhn V, D’Orsi ET, Johnson S, D’Orsi CJ, Adams AL, O’Neill WC: Breast arterial calcification: A marker of medial vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol 6: 377–382, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Lomashvili K, Garg P, O’Neill WC: Chemical and hormonal determinants of vascular calcification in vitro. Kidney Int 69: 1464–1470, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Giachelli CM: The emerging role of phosphate in vascular calcification. Kidney Int 75: 890–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leinster SJ, Whitehouse GH: Factors which influence the occurrence of vascular calcification in the breast. Br J Radiol 60: 457–458, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Nielsen BB, Holm NV: Calcification in breast arteries. The frequency and severity of arterial calcification in female breast tissue without malignant changes. Acta Pathol Microbiol Immunol Scand [A] 93: 13–16, 1985 [PubMed] [Google Scholar]
- 13.Topal U, Kaderli A, Topal NB, Ozdemir B, Yeşilbursa D, Cordan J, Ediz B, Aydinlar A: Relationship between the arterial calcification detected in mammography and coronary artery disease. Eur J Radiol 63: 391–395, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Iribarren C, Go AS, Tolstykh I, Sidney S, Johnston SC, Spring DB: Breast vascular calcification and risk of coronary heart disease, stroke, and heart failure. J Womens Health (Larchmt) 13: 381–389, discussion 390–392, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Crystal P, Crystal E, Leor J, Friger M, Katzinovitch G, Strano S: Breast artery calcium on routine mammography as a potential marker for increased risk of cardiovascular disease. Am J Cardiol 86: 216–217, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kemmeren JM, Beijerinck D, van Noord PA, Banga JD, Deurenberg JJ, Pameijer FA, van der Graaf Y: Breast arterial calcifications: association with diabetes mellitus and cardiovascular mortality. Work in progress. Radiology 201: 75–78, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Baum JK, Comstock CH, Joseph L: Intramammary arterial calcifications associated with diabetes. Radiology 136: 61–62, 1980 [DOI] [PubMed] [Google Scholar]
- 18.Morrison LB, Bogan IK: Calcification of the vessels in diabetes: A roentgraphic study of legs and feet. JAMA 92: 1424–1426, 1929 [Google Scholar]
- 19.Ferrier TM: Radiologically demonstrable arterial calcification in diabetes mellitus. Australas Ann Med 13: 222–228, 1964 [DOI] [PubMed] [Google Scholar]
- 20.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH: Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia 31: 16–23, 1988 [DOI] [PubMed] [Google Scholar]
- 21.Kemmeren JM, van Noord PAH, Beijerinck D, Fracheboud J, Banga J-D, van der Graaf Y: Arterial calcification found on breast cancer screening mammograms and cardiovascular mortality in women: The DOM Project. Doorlopend Onderzoek Morbiditeit en Mortaliteit. Am J Epidemiol 147: 333–341, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Taşkin F, Akdilli A, Karaman C, Unsal A, Köseoğlu K, Ergin F: Mammographically detected breast arterial calcifications: indicators for arteriosclerotic diseases? Eur J Radiol 60: 250–255, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Reddy J, Bilezikian JP, Smith SJ, Mosca L: Reduced bone mineral density is associated with breast arterial calcification. J Clin Endocrinol Metab 93: 208–211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sickles EA, Galvin HB: Breast arterial calcification in association with diabetes mellitus: Too weak a correlation to have clinical utility. Radiology 155: 577–579, 1985 [DOI] [PubMed] [Google Scholar]
- 25.Schmitt EL, Norbeck JM, Threatt B: Incidence of mammary intra-arterial calcification: An age-matched control study. South Med J 78: 1440–1442, 1985 [DOI] [PubMed] [Google Scholar]
- 26.Fiuza Ferreira EM, Szejnfeld J, Faintuch S: Correlation between intramammary arterial calcifications and CAD. Acad Radiol 14: 144–150, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Kataoka M, Warren R, Luben R, Camus J, Denton E, Sala E, Day N, Khaw K-T: How predictive is breast arterial calcification of cardiovascular disease and risk factors when found at screening mammography? AJR Am J Roentgenol 187: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Edmonds ME, Morrison N, Laws JW, Watkins PJ: Medial arterial calcification and diabetic neuropathy. Br Med J (Clin Res Ed) 284: 928–930, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumaine RL, Montalescot G, Steg PG, Ohman EM, Eagle K, Bhatt DL. REACH Registry Investigators: Renal function, atherothrombosis extent, and outcomes in high-risk patients. Am Heart J 158: 141–148, e1, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) Study Group: Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orth SR, Hallan SI: Smoking: a risk factor for progression of chronic kidney disease and for cardiovascular morbidity and mortality in renal patients—absence of evidence or evidence of absence? Clin J Am Soc Nephrol 3: 226–236, 2008 [DOI] [PubMed] [Google Scholar]