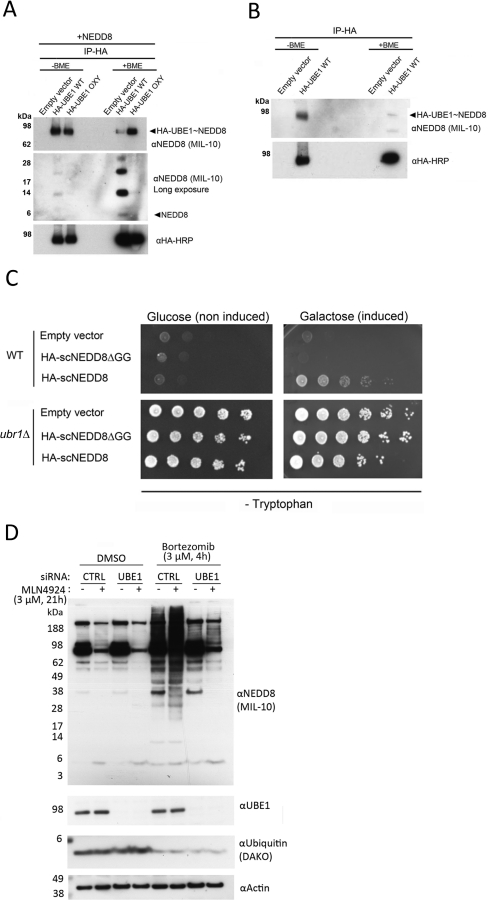
Figure 5. NEDD8 is present on the UBE1 catalytic cysteine in vivo, and NEDD8 overexpression attenuates proteasomal degradation of a reporter substrate.
(A) Untagged mature NEDD8 was co-expressed with WT HA–UBE1 as well as with HA–UBE1(C632S; OXY), which forms a non-reducible oxyester with the UBL. Immunoprecipitation reveals reducible thioester (WT) and non-reducible oxyester (OXY) adducts with NEDD8. In reducing conditions, free NEDD8 can be seen to fall off the WT HA–UBE1, but not the oxyester mutant, demonstrating that NEDD8 resides on the catalytic cysteine residue of this enzyme. Higher molecular mass forms of NEDD8 are also apparent after reduction from UBE1 WT, possibly corresponding to forms of NEDD8 more efficiently activated by UBE1. (B) The same experiment as described in (A) was performed for WT HA–UBE1, but without simultaneous NEDD8 overexpression, to investigate if endogenous NEDD8 would be available for activation upon UBE overexpression. A reducible thioester adduct with endogenous NEDD8 is apparent after immunopreciptiation (IP), demonstrating that endogenous NEDD8 can be activated when UBE1 is available. (C) scNEDD8 overexpression attenuates degradation of a Trp1 reporter substrate. Serial dilution of Trp1 reporter yeast carrying galactose-inducible HA–scNEDD8 or HA–scNEDD8ΔGG. Induction of HA–scNEDD8 restores growth on −TRP plates, indicating stabilization of the Trp1 reporter. Deletion of the yeast N-end rule E3 ligase Ubr1 stabilizes Trp1 and results in loss of tryptophan auxotrophy. (D) Bortezomib treatment induces atypical NEDDylation. U2OS cells were treated with 3 μM bortezomib or DMSO (vehicle) for 4 h, and with siRNA to UBE1 and MLN4924 as indicated. Widespread UBE1-dependent NEDDylation is apparent after bortezomib treatment. Molecular masses in kDa are shown to the left-hand side of the Western blots. CTRL, control.