Abstract
Objective:
To determine the prevalence and incidence of epilepsy among US Medicare beneficiaries aged 65 years old and over, and to compare rates across demographic groups.
Methods:
We performed a retrospective analysis of Medicare administrative claims for 2001–2005, defining prevalent cases as persons with ≥1 claim with diagnosis code 345.xx (epilepsy) or 2 or more with diagnosis code 780.3x (convulsion) ≥1 month apart, and incident cases as prevalent cases with 2 years immediately before diagnosis without such claims. Prevalence and incidence rates were calculated for the years 2003–2005 using denominators estimated from a 5% random sample of Medicare beneficiaries. Results were correlated with gender, age, and race.
Results:
We identified 282,661 per year on average during 2001–2005 (a total of 704,243 unique cases overall), and 62,182 incident cases per year on average during 2003–2005. Average annual prevalence and incidence rates were 10.8/1,000 and 2.4/1,000. Overall, rates were higher for black beneficiaries (prevalence 18.7/1,000, incidence 4.1/1,000), and lower for Asians (5.5/1,000, 1.6/1,000) and Native Americans (7.7/1,000, 1.1/1,000) than for white beneficiaries (10.2/1,000, 2.3/1,000). Incidence rates were slightly higher for women than for men, and increased with age for all gender and race groups.
Conclusions:
Epilepsy is a significant public health problem among Medicare beneficiaries. Efforts are necessary to target groups at higher risk, such as minorities or the very old, and to provide the care necessary to reduce the negative effects of epilepsy on quality of life.
Epilepsy is a serious detriment to the physical, cognitive, and social well-being of older adults. Understanding its epidemiology in this population is of great public health importance especially as the US health care system deals with increasing numbers of older adults with epilepsy in the upcoming years.1 Few estimates are available for epilepsy prevalence and incidence in older adults. Prevalence rates range from 13 to 50 per 1,000 depending on the population studied.2–5 Incidence rates range from 1.0 to 2.6 per 1,000 depending on age.6,7 These estimates are from geographically localized populations in areas such as the Bronx, NY, or Rochester, MN.6,7 It is not clear whether they can generalize to the larger US population 65 years old and older, or what the rates are in different age, gender, and racial groups.
The Medicare administrative claims database includes health care utilization data for over 95% of the American population above age 65, and is not restricted by treatment location. It represents a unique opportunity to obtain generalizable estimates of epilepsy prevalence and incidence and to assess the burden of epilepsy among older adults. The goals of this study were to determine the frequency of epilepsy in the Medicare population across demographic groups.
METHODS
To conduct this study, we first identified a group of Medicare beneficiaries aged 65 and older with claims for seizures and epilepsy; i.e., those who had at least one claim filed by noninstitutional providers (e.g., physicians) with International Classification of Disease–Version 9–Clinical Modification (ICD-9) codes for epilepsy (345.xx) or for seizures (780.3x) during the period 2001–2005. We limited this group to beneficiaries who 1) had Medicare because of older age (and not disability or end-stage renal disease); 2) were continuously enrolled in Medicare part A and B; and 3) did not have managed care plans (because administrative claims for beneficiaries in managed care plans are not available). For this group, we obtained administrative claims for inpatient, outpatient, and physician visits in the period 2001–2006 to ensure at least 1 year of follow-up for all.
Prevalent cases of epilepsy in each year from 2001 to 2005 were defined using a claim-based epilepsy diagnosis. That is, they were those of beneficiaries who had either one claim with ICD-9 code for “epilepsy” (345.xx) or 2 claims with ICD-9 codes for “convulsions” (780.3x) at least 30 days apart among claims for inpatient stays, outpatient or physician visits for that year. We eliminated those cases with more frequent seizure claims to exclude those with only acute symptomatic seizures, i.e., caused by a transient condition. This is based in part on the consideration that posttraumatic seizures, for example, are typically divided into “early posttraumatic seizures” occurring often within the first 2 weeks after injury and “late posttraumatic seizures”: patients with only early posttraumatic seizures are not diagnosed with epilepsy. We opted to not exclude 780.31 and 780.32 codes that indicate febrile seizures because these codes should not apply to our older population. We did not consider beneficiaries to be cases in 1 calendar year if they met the claim-based epilepsy diagnosis based on 2 seizure claims that crossed the calendar year. We also excluded beneficiaries with claims for seizures that did not correspond to a physician evaluation or management or to a medical procedure (Berenson-Eggers Type of Service codes M for evaluation and management and P for procedures), thereby excluding claims submitted by ambulance providers, for example, whose diagnosis of a seizure could be less accurate than that of a physician.
Incident cases were a subset of prevalent cases and were defined in the same way as above but requiring in addition that beneficiaries had a “clean period” of 2 years: that is, a beneficiary had no claims with the 345.xx or the 780.3x codes for a period of 2 years before the claim-based epilepsy diagnosis. We excluded cases whose first event included a code of 345.x1, which designates intractable epilepsy. To allow for all beneficiaries to have at least 2 years of claims data before a diagnosis of epilepsy, incident cases were identified for the years 2003–2005.
Based on a study by Holden et al.,8 we calculated the potential positive predictive value (pPPV) of our claim-based epilepsy diagnosis. In particular, Holden et al. found that 1) cases defined based on only one claim with ICD-9 345.xx had a PPV of 38.5%, 2) cases defined based on 2–3 claims with ICD-9 codes 345.xx and/or 780.3x had a PPV of 60%–69%, and 3) cases defined based on 4 or more claims with ICD-9 codes 345.xx and/or 780.3x had a PPV of more than 78%. We calculated a lower level pPPV considering how many cases on average each year were classified as such based on 1, 2–3, and 4 or more claims with the relevant ICD-9 codes. We calculated a higher level pPPV considering the proportion of cases that also met the definition of epilepsy in at least another year and thus, considering the number of claims with relevant ICD-9 codes over all years.
Analysis.
Prevalence and incidence rates were determined for the years 2003 to 2005. Denominators were obtained using a 5% random sample of all US Medicare beneficiaries age 65 and older to which we applied the same restrictions used to define the group of beneficiaries with seizures (no end-stage renal disease, continuous enrollment in parts A and B, not in managed care plans).
Prevalence and incidence rates were calculated by race, gender, and age group. Race, gender, and age information was obtained from Medicare files. Racial groups, using Medicare terminology, included white, black, Asian, Native American, and other. Age at the time of the epilepsy claim–based diagnosis was determined using the date of the first coding of seizure or epilepsy. χ2 tests were used to assess significant differences in rates across groups.
Sensitivity analysis.
In sensitivity analyses we relaxed some of the restrictions imposed on our selected groups. We determined the number of prevalent cases among beneficiaries aged 65 and older even if they were not continuously enrolled in Medicare part A and B, or even if they had managed care plans in the study period (unrestricted group with claims for seizures or epilepsy, n = 1,831,582). Furthermore, among beneficiaries observed for all years from 2001 to 2005, we identified incident cases in the year 2005 using different lengths of the clean period, i.e., 2, 3, and 4 years.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Board of the University of Alabama at Birmingham.
RESULTS
We identified 1,463,723 beneficiaries with claims for epilepsy or seizures in 2001–2005. Among them, we identified a total of 704,243 epilepsy cases during 2001–2005 and 186,547 incident cases during 2003–2005 (table 1). On average, we identified 282,661 cases of beneficiaries with epilepsy each year from 2001 to 2005: of these, 62,182 on average in 2003 to 2005 were classified as incident cases of epilepsy.
Table 1.
Characteristics of a group of Medicare beneficiaries in fee-for-service plans with claims for epilepsy or seizures, prevalent and incident cases of epilepsy, and of a random sample of Medicare beneficiaries
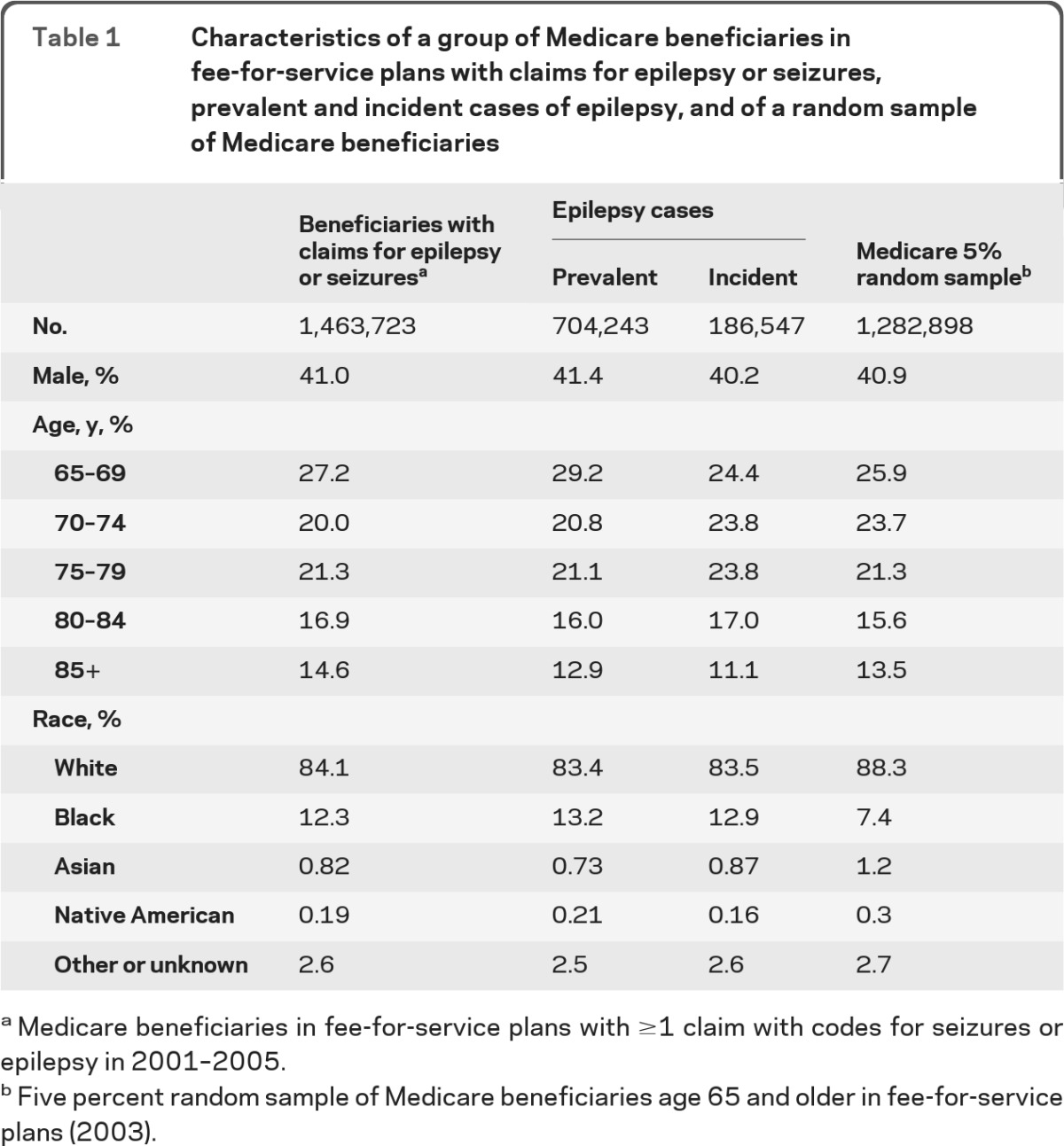
Medicare beneficiaries in fee-for-service plans with ≥1 claim with codes for seizures or epilepsy in 2001–2005.
Five percent random sample of Medicare beneficiaries age 65 and older in fee-for-service plans (2003).
We estimated that the pPPV for this study ranged from 68.9% to 74.9% on average. Fewer than 9% of cases identified each year were classified as such based on only one claim with an ICD-9 code of 345.xx, and half of these met the epilepsy definition in at least one other year. Another 55.6% were classified based on 2–3 claims with seizure or epilepsy ICD-9 codes; of them, 83% met the epilepsy definition in at least one other year. The rest were classified as cases based on 4 or more claims with relevant ICD-9 codes, and of them about 87% met the epilepsy diagnosis in at least one other year. Overall, only 21% of cases on average had fewer than 4 claims for seizure or epilepsy in only 1 year. In addition, only 2% of cases were defined as such based on ICD-9 codes 780.31 and 780.32.
Compared to beneficiaries with claims for epilepsy and seizures, prevalent and incident epilepsy cases had smaller representations of the oldest age group, and slightly higher representation of black beneficiaries (table 1). Compared to the 5% random sample of Medicare beneficiaries in 2003, the group of beneficiaries with claims for epilepsy and seizures, that of prevalent and incident cases had a higher proportion of black beneficiaries and a smaller proportion of Asians and Native Americans.
The average annual prevalence rate of epilepsy among Medicare beneficiaries for 2003–2005 was 10.8 per 1,000 and the average incidence rate was 2.4 per 1,000 (table 2). The incidence rate increased with age, and women had a slightly higher rate than men. Black beneficiaries had significantly higher prevalence and incidence rates than white beneficiaries, while Asians and Native Americans had lower rates (table 2).
Table 2.
Prevalence and incidence rates (per 1,000) of epilepsy: Medicare beneficiaries in fee-for-service plans, 2003–2005
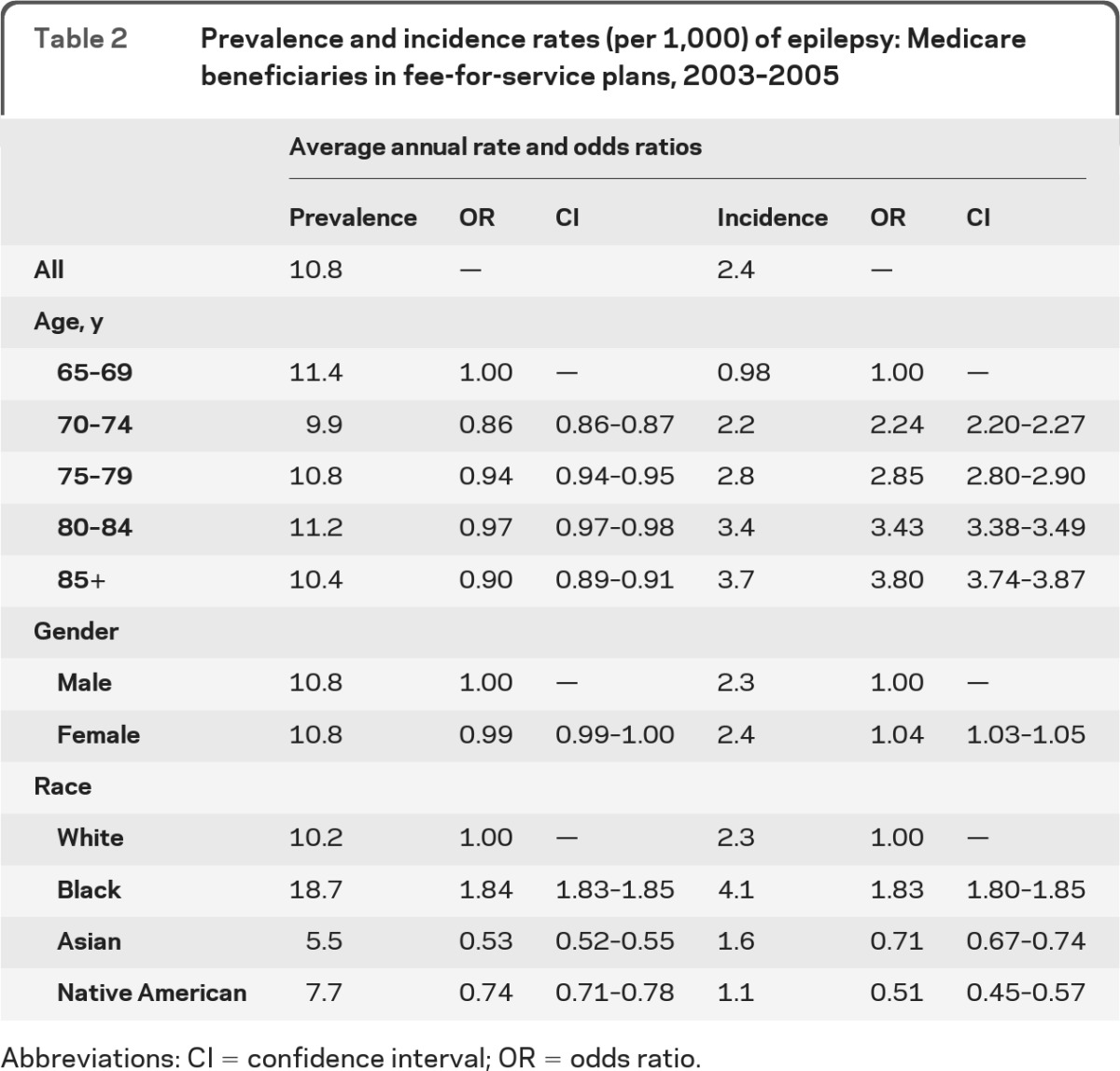
Abbreviations: CI = confidence interval; OR = odds ratio.
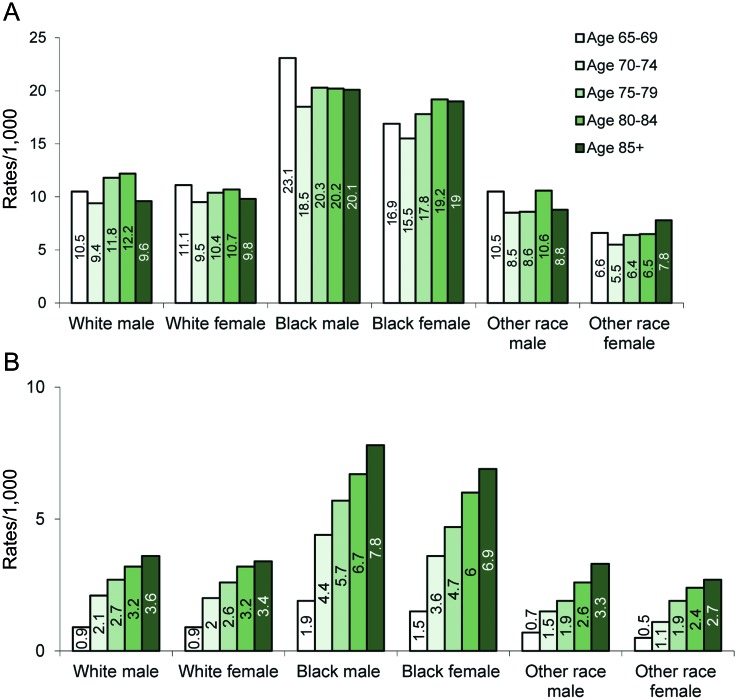
The figure shows the trends in prevalence and incidence rates by age within each race and gender group. In general, the highest rates were among black males followed by black females for each age group (figure, A and B). Incidence rates increased with age for all groups, with those for black males being the highest for each age group (figure, B). In addition, although the overall incidence rate was higher for female than for male beneficiaries, age and race-specific rates were not. Female beneficiaries, however, were more likely to be older than males (p < 0.0001): for example, 20.3% of them were 85 years old or older compared with 12.1% of male beneficiaries (data not shown).
Figure. Frequency of epilepsy by race, gender, and age.
(A) Average prevalence rates of epilepsy by race and gender groups and by age at first observed seizure for Medicare beneficiaries with a claim-based diagnosis of epilepsy (2003–2005). (B) Average incidence rates of epilepsy by race and gender groups and by age at first observed seizure for Medicare beneficiaries with a claim-based diagnosis of epilepsy (2003–2005). Other race indicates Asian or Native American.
Sensitivity analysis.
In the unrestricted group of 1,813,582 beneficiaries with claims for epilepsy or seizures with no restrictions on continuous enrollment in Medicare part A and B and on membership in fee-for-service plans, 41.3% were male, 81.5% were white, 13.6% were black, and 13% were older than 85 years. In this group, we identified 848,527 prevalent epilepsy cases and the average annual prevalence rate was calculated to be 11.5 per 1,000 (vs 10.8 per 1,000 when using the restricted group). Among beneficiaries observed for all years from 2001 to 2005, we found that the incidence rate for the year 2005 declined as the clean period became longer. This rate was 2.3 per 1,000 if the length of the clean period was 2 years, 2.0 per 1,000 if it was 3 years, and 1.8 per 1,000 if it was 4 years.
DISCUSSION
A mean prevalence rate between 10.8 and 11.5 per 1,000 and an annual incidence rate of 2.4 per 1,000 indicate that epilepsy is common among Medicare beneficiaries aged 65 and over. The magnitude of these rates indicates a significant and unrecognized public health issue. Other diseases of similar frequency receive more attention; for example, the incidence of colorectal cancer is about 2.5 per 1,000 in the population aged 65 and older.9 Even when considering that our case-finding algorithm may identify true cases of epilepsy only 70% of the time, the numbers of cases remain considerable at more than 197,000 prevalent cases and more than 43,000 potential incident cases every year.
Our findings, based on a nationwide database of over 40 million people, concur with the results of studies of smaller population groups,3,7,10 which also indicate that rates of epilepsy in the older population are higher than those for the general US population of 7.1 per 1,000 for all age groups.11 The incidence of epilepsy in our study was higher than the incidence rate reported in previous studies that included older people.2,7 One reason may be because many previous studies were of groups less likely to be representative of the whole US population, including studies of small geographic areas2,7,12–14 or relatively homogeneous ethnic or socioeconomic groups.4,10,12 Another reason is that our incident cases may not all be true cases of new-onset epilepsy and that we may have overestimated the incidence. Indeed, our sensitivity analysis indicated that using longer clean periods leads to identifying fewer cases as incident. Incidence rates increased significantly with age in all race and gender groups. The correlation of age and incidence has been reported previously. In the Bronx study, epilepsy incidence rose dramatically with age: per 1,000 patient-years, it was 0.26 for ages 60–74, and 1.01 for those 75–89.7 A similar correlation was also found in the Rochester study.10
Prevalence and incidence rates were highest among black beneficiaries, in particular black men. This is consistent with earlier studies,13,15 and has been ascribed to a higher risk of hypertension and stroke. However, there may be other influences predisposing older African Americans to seizures: in one study, race was found to be an independent factor when stroke was accounted for.7 The racial difference between African Americans and whites was not found in other populations, such as among members of an urban health maintenance organization (HMO) in Houston, TX.16 The population in the Texas study differed from ours because it included mostly adults younger than 65 who were healthy and employed. Furthermore, we find the prevalence among Native American beneficiaries to be lower than that reported among residents of the Navajo Nation who received care from the Indian Health Services.4 The Navajo data may not be representative of Native Americans residing elsewhere who receive care from Medicare providers. In addition, we found a low frequency of epilepsy among Asian beneficiaries. This is in line with lower incidence rates found for Asians compared to whites in the Texas HMO.16
Women were slightly more likely to have incident cases of epilepsy than men; this difference achieved statistical significance due to the large study population. Other studies of older adults have failed to find differences by gender,7,12 and some studies including subjects of all ages have found a higher risk for males.2,4,13–14 In our study, the observed gender effect was due to the age difference between male and female beneficiaries. Because they lived longer than men, women were more likely to experience the higher rates of epilepsy at older ages. Within the same age group, rates for women were lower than for men.
The frequency of epilepsy must be contemplated in light of the impact of seizures on the lives of older adults. Seizures not only cause injuries, high utilization of medical resources, and death, but also create serious social restrictions, reduce income, and lower quality of life.17 Older people are likely to have serious injuries from seizures such as falls and broken bones, and are more prone to have side effects of antiepileptic medications.18 Moreover, postictal confusional states may last longer, and if status epilepticus supervenes, it is much more likely to be fatal.19 Older adults with epilepsy are disproportionately subject to cognitive decline.20 There are several potential reasons for older adults to be more susceptible to epilepsy, with the occurrence of comorbidities such as stroke and dementia being among the most commonly identifiable causes.3,10,21–23 Despite the burden associated with this disease, currently, very little is known about the extent to which older Medicare beneficiaries receive appropriate evaluation and care for their seizures.
There are some limitations to our data. The most important is that our estimates are based on data on health care utilization, and thus on whether beneficiaries sought and obtained medical care. For example, we may have missed less severe cases that did not require medical attention. Moreover, the most common seizure type in the elderly is complex partial,18 which may be difficult to recognize since these seizures may consist merely of blank staring. Furthermore, data are accurate if diagnosis codes are used accurately. The codes for epilepsy, seizures, and convulsions are confusing, and physicians may be unsure of which one to select for a particular patient. Patients may also receive an epilepsy diagnosis code in error if they have syncope, psychogenic unresponsiveness, or other nonepileptic phenomena. In one study, the most common reason for identifying a false positive epilepsy case was due to the use of the ICD-9 code for epilepsy, 345.xx, instead of the ICD-9 code for migraine, 346.xx.4 Our definition of epilepsy was more conservative than definitions used by others as it required one or more 345.xx codes or 2 or more 780.3x codes, and the 780.3x codes had to occur at least 30 days apart. Several previous studies required only one or more of either code without time constraints7,24; therefore, our method may have been less sensitive but more specific than others. Furthermore, information on filled antiepileptic drugs was not available in our database: adding such information could have improved our ability to identify true cases of epilepsy.8 Finally, as mentioned above, our methods may have overestimated incident cases by including patients who had a new diagnosis within our time window, but who may have had seizures more than 2 years earlier with or without intervening claims. Expanding the “clean” period by 1 year reduced the incidence rate by about 10%.
In this study we found that a significant number of Medicare beneficiaries is affected by epilepsy and its associated poorer quality of life, especially African American men and those older than 80. Understanding the epidemiology of epilepsy in older adults is important for public health officials to understand whether resources are in place to address the needs of this population group. Physicians must be made aware of the extent of the problem: given its prevalence and the often subtle nature of seizures in older patients, they must give the possibility of an epilepsy diagnosis appropriate consideration. They also must be educated about the best care for older adults with epilepsy: due to the coexistence of numerous other chronic conditions and drug treatments in this population, treatment is particularly challenging. More research is needed to determine if older adults of all race and age groups receive appropriate care for epilepsy and are, thus, given the opportunity to enhance their quality of life.
Supplementary Material
GLOSSARY
- HMO
health maintenance organization
- ICD
International Classification of Disease
- pPPV
potential positive predictive value.
Footnotes
Editorial, page 444
AUTHOR CONTRIBUTIONS
Biostatistical analysis: performed by Y. Kim under supervision of Drs. Richman and Pisu, none of whom report any disclosures. Dr. Faught: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Richman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Martin: drafting/revising the manuscript, study concept or design. Dr. Funkhouser: drafting/revising the manuscript, analysis or interpretation of data. Dr. Foushee: study concept or design, acquisition of data. Dr. Kratt: drafting/revising the manuscript, acquisition of data, study supervision. Y. Kim: analysis or interpretation of data, statistical analysis. K. Clements: study concept or design, analysis or interpretation of data, subject matter expert for the ICD-9-CM codes. N.J. Cohen: analysis or interpretation of data, subject interview. D. Adoboe: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Knowlton: analysis or interpretation of data. study supervision. Dr. Pisu: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding.
DISCLOSURE
Dr. Faught serves on scientific advisory boards for Eisai Inc., Lundbeck, Inc., Supernus Pharmaceuticals, Inc., Sunovion Pharmaceuticals Inc., UCB, and Zogenix, Inc.; has received funding for travel from Johnson & Johnson; serves as Associate Editor for Frontiers in Neurology; and receives research support from GlaxoSmithKline, Cyberonics, Inc., Johnson & Johnson, SCHWARZ PHARMA, and Marinus Pharmaceuticals, Inc. Dr. Richman receives research support from Pfizer Inc, the U.S. Veterans Administration HSR&D, the U.S. Centers for Disease Control, the NIH (NIDA, NCI, NIA), AHRQ, DHHS, and the American Academy of Family Physicians. Dr. Martin serves on the editorial board of Epilepsy & Behavior; serves as a consultant for Pfizer Inc; and receives research support from the NIH (NICHD/NCMMR, NIA) and the U.S. Centers for Disease Control. Dr. Funkhouser, Dr. Foushee, Dr. Kratt, Y. Kim, K. Clements, N.J. Cohen, and D. Adoboe report no disclosures. Dr. Knowlton serves as a consultant for Excel-Tech, Ltd, a division of Natus Medical Incorporated; serves on the speakers' bureau for; UCB; and receives research support from Eisai Inc. and the NIH/NINDS. Dr. Pisu receives research support from the U.S. Centers for Disease Control and Prevention, AHRQ, and the NIH.
REFERENCES
- 1. Rowan AJ. What we owe our seniors with epilepsy: thoughts on quality of life. Epilepsy Behav 2000; 1: 215– 216 [DOI] [PubMed] [Google Scholar]
- 2. Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993; 34: 453– 468 [DOI] [PubMed] [Google Scholar]
- 3. Holden EW, Thanh Nguyen H, Grossman E, et al. Estimating prevalence, incidence, and disease-related mortality for patients with epilepsy in managed care organizations. Epilepsia 2005; 46: 311– 319 [DOI] [PubMed] [Google Scholar]
- 4. Parko K, Thurman DJ. Prevalence of epilepsy and seizures in the Navajo Nation 1998–2002. Epilepsia 2009; 50: 2180– 2185 [DOI] [PubMed] [Google Scholar]
- 5. Schachter SC, Cramer GW, Thompson GD, Chaponis RJ, Mendelson MA, Lawhorne L. An evaluation of antiepileptic drug therapy in nursing facilities. J Am Geriatr Soc 1998; 46: 1137– 1141 [DOI] [PubMed] [Google Scholar]
- 6. Hauser WA. Seizure disorders: the changes with age. Epilepsia 1992; 33 suppl 4: S6– S14 [DOI] [PubMed] [Google Scholar]
- 7. Hussain SA, Haut SR, Lipton RB, Derby C, Markowitz SY, Shinnar S. Incidence of epilepsy in a racially diverse, community-dwelling, elderly cohort: results from the Einstein aging study. Epilepsy Res 2006; 71: 195– 205 [DOI] [PubMed] [Google Scholar]
- 8. Holden EW, Grossman E, Nguyen HT, et al. Developing a computer algorithm to identify epilepsy cases in managed care organizations. Dis Manag 2005; 8: 1– 14 [DOI] [PubMed] [Google Scholar]
- 9. National Cancer Institute. Surveillance Epidemiology and End Results (SEER) Cancer Statistics Review 1975–2008. Available at: www.SEER.cancer.gov Accessed May 19, 2011
- 10. Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 1996; 71: 576– 586 [DOI] [PubMed] [Google Scholar]
- 11. Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology 2007; 68: 326– 337 [DOI] [PubMed] [Google Scholar]
- 12. Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia 1996; 37: 224– 229 [DOI] [PubMed] [Google Scholar]
- 13. Haerer AF, Anderson DW, Schoenberg BS. Prevalence and clinical features of epilepsy in a biracial United States population. Epilepsia 1986; 27: 66– 75 [DOI] [PubMed] [Google Scholar]
- 14. Luhdorf K, Jensen LK, Plesner AM. Epilepsy in the elderly: incidence, social function, and disability. Epilepsia 1986; 27: 135– 141 [DOI] [PubMed] [Google Scholar]
- 15. Hollingsworth J, Alsikafi M, Coombs D. The Prevalence of the Development Disabilities in Alabama: Findings from the Research: Mental Retardation, Cerebral Palsy and Epilepsy in Alabama: A Sociological Analysis. Tuscaloosa: The University of Alabama Press; 1971: 88–110 [Google Scholar]
- 16. Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999; 40: 502– 506 [DOI] [PubMed] [Google Scholar]
- 17. Theodore WH, Spencer SS, Wiebe S, et al. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia 2006; 47: 1700– 1722 [DOI] [PubMed] [Google Scholar]
- 18. Ramsay RE, Rowan AJ, Pryor FM. Special considerations in treating the elderly patient with epilepsy. Neurology 2004; 62 5 suppl 2: S24– S29 [DOI] [PubMed] [Google Scholar]
- 19. Faught E. Epidemiology and drug treatment of epilepsy in elderly people. Drugs Aging 1999; 15: 255– 269 [DOI] [PubMed] [Google Scholar]
- 20. Hermann B, Seidenberg M, Sager M, et al. Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia 2008; 49: 731– 740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Arch Neurol 2002; 59: 195– 201 [DOI] [PubMed] [Google Scholar]
- 22. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000; 57: 1617– 1622 [DOI] [PubMed] [Google Scholar]
- 23. Rowan AJ, Ramsay RE, Collins JF, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology 2005; 64: 1868– 1873 [DOI] [PubMed] [Google Scholar]
- 24. Leppik IE. Treatment of epilepsy in the elderly. Curr Treat Options Neurol 2008; 10: 239– 245 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.