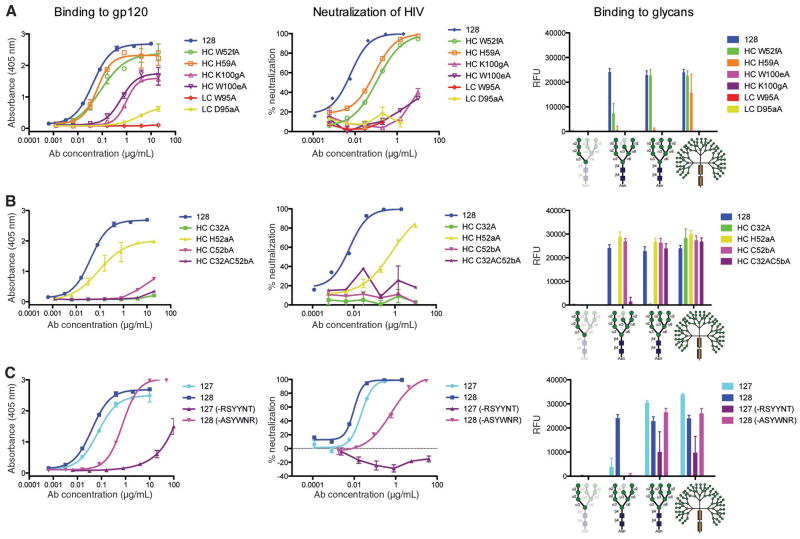
Fig. 3.
Effect of PGT 128 paratope mutations in the individual glycan subsites on neutralization of HIV-1JR-FL and glycan binding. Binding of PGT 128 mutants to gp120 was tested by ELISA (left panel) or to glycans on the high mannose glycan microarray (right panel). (A) Mutation of select residues in the primary glycan binding site (Man8/9) that recognizes the N332 glycan. Residues (HC, heavy chain; LC, light chain) that disrupt the formation of the hydrophobic core of the binding site (VH K100gA, W100eA, and VL W95A) or disrupt hydrogen bonding to terminal mannose residues (VH H59A and VL D95aA) compromise neutralization (middle panel), as well as gp120 and glycan binding. (B) Mutation of select residues interacting with the secondary glycan binding site that recognizes the N301 glycan. Mutation of VH H52aA results in a decrease in gp120 binding and neutralization, while disruption of the CDR H1–H2 disulfide (VH C32A, C52bA, or double mutant) greatly compromises both gp120 binding and neutralization. There is much less effect on the glycan array which primarily reflects binding to the primary glycan binding site. A complete list of paratope mapping, as well as the effect on gp120 binding, is provided in Table S3. (C) Contribution of the 6-residue CDR H2 insert to neutralization and glycan binding. PGT 128 retains the ability to bind Man8/9 and neutralize to a lesser extent on deletion of the insert, whereas PGT 127 no longer neutralizes, although still has some ability to bind Man8/9. Swapping of the insert between 127 and 128 allows 128 to retain some binding and neutralization, but substantially reduces binding and abrogates neutralization when the PGT 128 H2 insert is transplanted onto PGT 127.