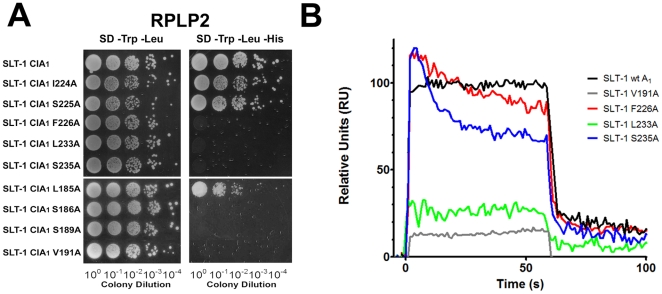
Figure 4. The interaction of the A1 chain of SLT-1 with the ribosomal stalk protein P2 and the C-terminal peptide SDDDMGFGLFD also involves hydrophobic residues within the A1 chain.
(A) Bait vectors expressing either a catalytically inactive variant of the wild-type SLT-1 A1 domain (CIA1) or one of the hydrophobic mutants were co-transformed in the yeast strain AH109 with a prey vector expressing ribosomal protein P2. The transformed yeast cells were plated on SD agar −Trp/−Leu. The resulting yeast colonies were grown overnight, and spotted (10 µl) as 10-fold serial dilutions onto SD medium lacking Trp and Leu to select for the presence of each plasmid followed by spotting on SD media lacking Trp, Leu, and His to select for interacting partners. (B) SPR profiles (plotted at 15 µM) demonstrate that hydrophobic mutants F226A and S235A in the SLT-1 A1 chain have a minor effect on the binding to the conserved peptide SDDDMGFGLFD and the SLT-1 V191A and L233A A1 chain mutants cause a drastic decrease in binding. Experiments were performed in triplicate.